Input interpretation
carbonate anion | hypomanganate anion | formate anion
Structure diagrams
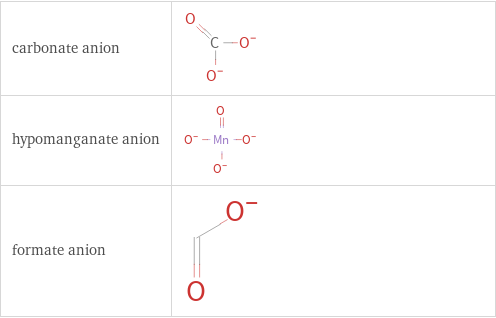
Structure diagrams
General properties
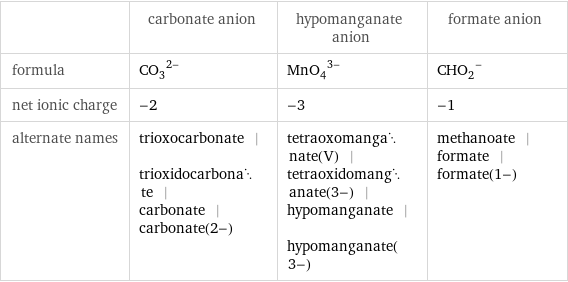
| carbonate anion | hypomanganate anion | formate anion formula | (CO_3)^(2-) | (MnO_4)^(3-) | (CHO_2)^- net ionic charge | -2 | -3 | -1 alternate names | trioxocarbonate | trioxidocarbonate | carbonate | carbonate(2-) | tetraoxomanganate(V) | tetraoxidomanganate(3-) | hypomanganate | hypomanganate(3-) | methanoate | formate | formate(1-)
Ionic radii
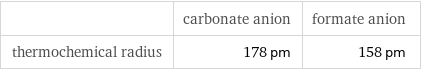
| carbonate anion | formate anion thermochemical radius | 178 pm | 158 pm
Units

Other properties
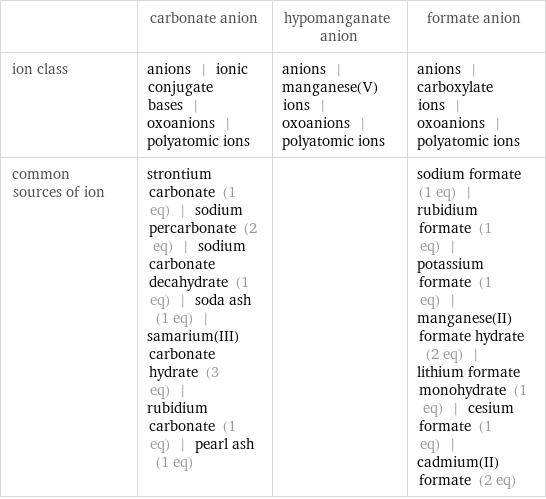
| carbonate anion | hypomanganate anion | formate anion ion class | anions | ionic conjugate bases | oxoanions | polyatomic ions | anions | manganese(V) ions | oxoanions | polyatomic ions | anions | carboxylate ions | oxoanions | polyatomic ions common sources of ion | strontium carbonate (1 eq) | sodium percarbonate (2 eq) | sodium carbonate decahydrate (1 eq) | soda ash (1 eq) | samarium(III) carbonate hydrate (3 eq) | rubidium carbonate (1 eq) | pearl ash (1 eq) | | sodium formate (1 eq) | rubidium formate (1 eq) | potassium formate (1 eq) | manganese(II) formate hydrate (2 eq) | lithium formate monohydrate (1 eq) | cesium formate (1 eq) | cadmium(II) formate (2 eq)