Input interpretation

non-polar solvents | element types
Summary
carbon | chlorine | hydrogen | oxygen
Periodic table location
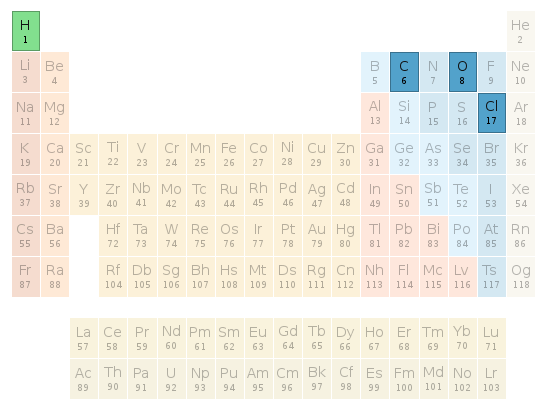
Periodic table location
Images
Images
Basic elemental properties
![| atomic symbol | atomic number | short electronic configuration carbon | C | 6 | [He]2s^22p^2 chlorine | Cl | 17 | [Ne]3s^23p^5 hydrogen | H | 1 | 1s^1 oxygen | O | 8 | [He]2s^22p^4 | Aufbau diagram | block | group carbon | 2p 2s | p | 14 chlorine | 3p 3s | p | 17 hydrogen | 1s | s | 1 oxygen | 2p 2s | p | 16 | period | atomic mass | half-life carbon | 2 | 12.011 u | (stable) chlorine | 3 | 35.45 u | (stable) hydrogen | 1 | 1.008 u | (stable) oxygen | 2 | 15.999 u | (stable)](../image_source/b0a12bf4ea2163e0a06ff9fda9408fe9.png)
| atomic symbol | atomic number | short electronic configuration carbon | C | 6 | [He]2s^22p^2 chlorine | Cl | 17 | [Ne]3s^23p^5 hydrogen | H | 1 | 1s^1 oxygen | O | 8 | [He]2s^22p^4 | Aufbau diagram | block | group carbon | 2p 2s | p | 14 chlorine | 3p 3s | p | 17 hydrogen | 1s | s | 1 oxygen | 2p 2s | p | 16 | period | atomic mass | half-life carbon | 2 | 12.011 u | (stable) chlorine | 3 | 35.45 u | (stable) hydrogen | 1 | 1.008 u | (stable) oxygen | 2 | 15.999 u | (stable)
Table
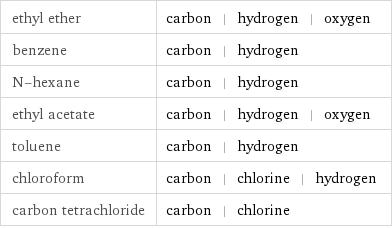
ethyl ether | carbon | hydrogen | oxygen benzene | carbon | hydrogen N-hexane | carbon | hydrogen ethyl acetate | carbon | hydrogen | oxygen toluene | carbon | hydrogen chloroform | carbon | chlorine | hydrogen carbon tetrachloride | carbon | chlorine
Thermodynamic properties
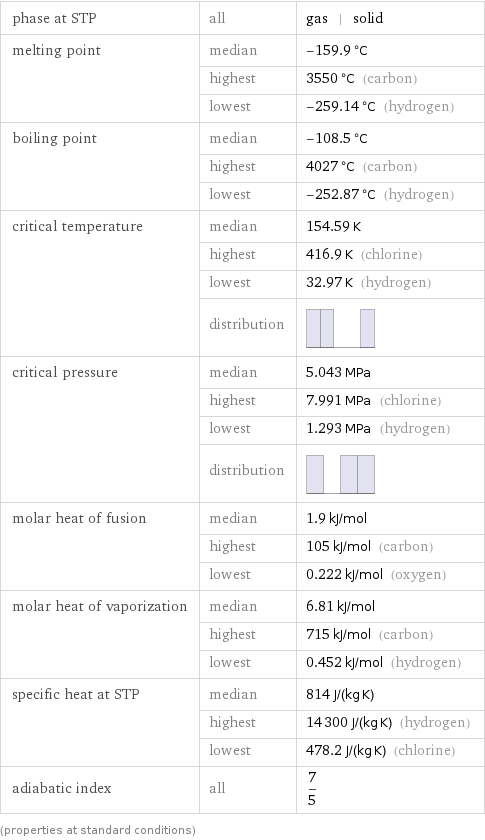
phase at STP | all | gas | solid melting point | median | -159.9 °C | highest | 3550 °C (carbon) | lowest | -259.14 °C (hydrogen) boiling point | median | -108.5 °C | highest | 4027 °C (carbon) | lowest | -252.87 °C (hydrogen) critical temperature | median | 154.59 K | highest | 416.9 K (chlorine) | lowest | 32.97 K (hydrogen) | distribution | critical pressure | median | 5.043 MPa | highest | 7.991 MPa (chlorine) | lowest | 1.293 MPa (hydrogen) | distribution | molar heat of fusion | median | 1.9 kJ/mol | highest | 105 kJ/mol (carbon) | lowest | 0.222 kJ/mol (oxygen) molar heat of vaporization | median | 6.81 kJ/mol | highest | 715 kJ/mol (carbon) | lowest | 0.452 kJ/mol (hydrogen) specific heat at STP | median | 814 J/(kg K) | highest | 14300 J/(kg K) (hydrogen) | lowest | 478.2 J/(kg K) (chlorine) adiabatic index | all | 7/5 (properties at standard conditions)
Material properties
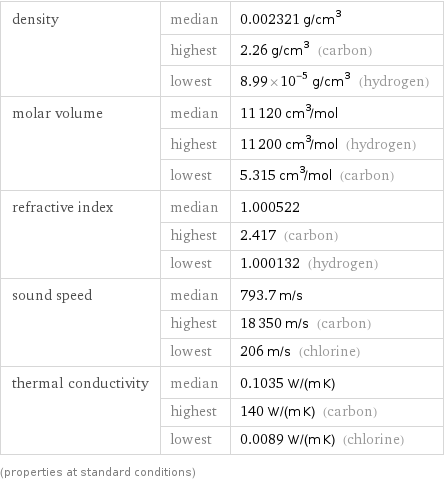
density | median | 0.002321 g/cm^3 | highest | 2.26 g/cm^3 (carbon) | lowest | 8.99×10^-5 g/cm^3 (hydrogen) molar volume | median | 11120 cm^3/mol | highest | 11200 cm^3/mol (hydrogen) | lowest | 5.315 cm^3/mol (carbon) refractive index | median | 1.000522 | highest | 2.417 (carbon) | lowest | 1.000132 (hydrogen) sound speed | median | 793.7 m/s | highest | 18350 m/s (carbon) | lowest | 206 m/s (chlorine) thermal conductivity | median | 0.1035 W/(m K) | highest | 140 W/(m K) (carbon) | lowest | 0.0089 W/(m K) (chlorine) (properties at standard conditions)
Reactivity
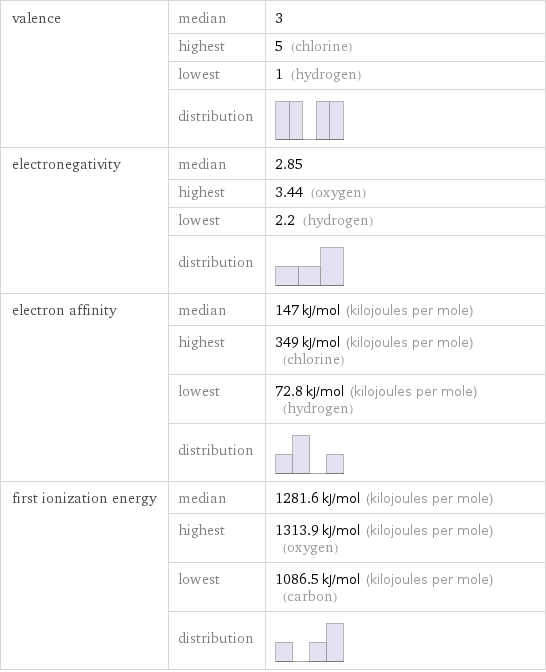
valence | median | 3 | highest | 5 (chlorine) | lowest | 1 (hydrogen) | distribution | electronegativity | median | 2.85 | highest | 3.44 (oxygen) | lowest | 2.2 (hydrogen) | distribution | electron affinity | median | 147 kJ/mol (kilojoules per mole) | highest | 349 kJ/mol (kilojoules per mole) (chlorine) | lowest | 72.8 kJ/mol (kilojoules per mole) (hydrogen) | distribution | first ionization energy | median | 1281.6 kJ/mol (kilojoules per mole) | highest | 1313.9 kJ/mol (kilojoules per mole) (oxygen) | lowest | 1086.5 kJ/mol (kilojoules per mole) (carbon) | distribution |
Atomic properties
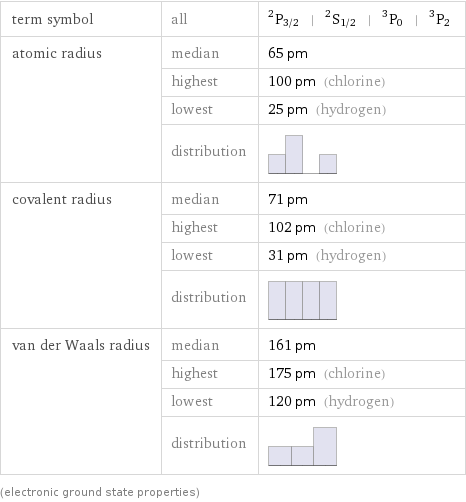
term symbol | all | ^2P_(3/2) | ^2S_(1/2) | ^3P_0 | ^3P_2 atomic radius | median | 65 pm | highest | 100 pm (chlorine) | lowest | 25 pm (hydrogen) | distribution | covalent radius | median | 71 pm | highest | 102 pm (chlorine) | lowest | 31 pm (hydrogen) | distribution | van der Waals radius | median | 161 pm | highest | 175 pm (chlorine) | lowest | 120 pm (hydrogen) | distribution | (electronic ground state properties)
Abundances
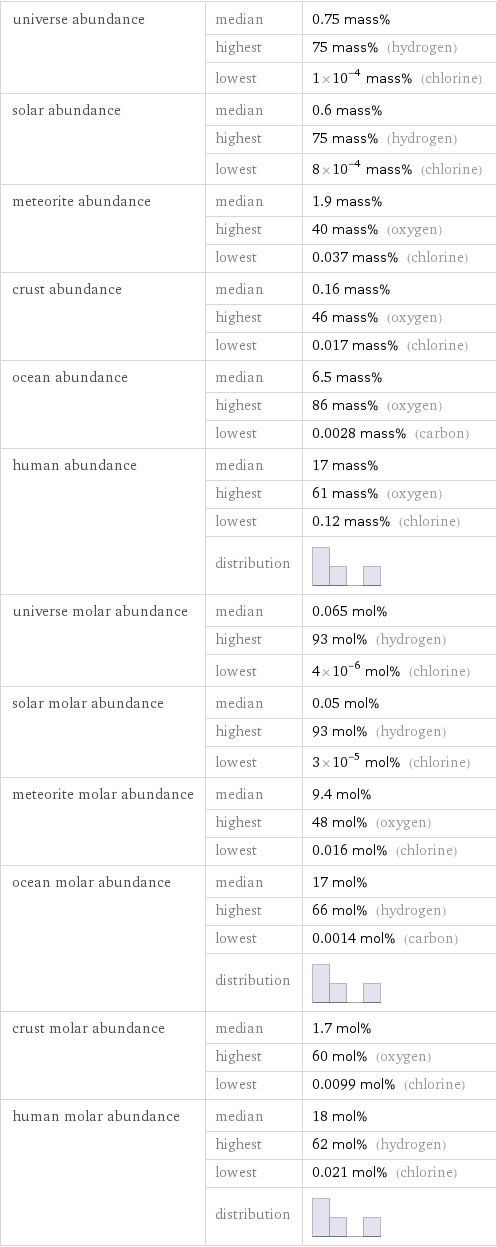
universe abundance | median | 0.75 mass% | highest | 75 mass% (hydrogen) | lowest | 1×10^-4 mass% (chlorine) solar abundance | median | 0.6 mass% | highest | 75 mass% (hydrogen) | lowest | 8×10^-4 mass% (chlorine) meteorite abundance | median | 1.9 mass% | highest | 40 mass% (oxygen) | lowest | 0.037 mass% (chlorine) crust abundance | median | 0.16 mass% | highest | 46 mass% (oxygen) | lowest | 0.017 mass% (chlorine) ocean abundance | median | 6.5 mass% | highest | 86 mass% (oxygen) | lowest | 0.0028 mass% (carbon) human abundance | median | 17 mass% | highest | 61 mass% (oxygen) | lowest | 0.12 mass% (chlorine) | distribution | universe molar abundance | median | 0.065 mol% | highest | 93 mol% (hydrogen) | lowest | 4×10^-6 mol% (chlorine) solar molar abundance | median | 0.05 mol% | highest | 93 mol% (hydrogen) | lowest | 3×10^-5 mol% (chlorine) meteorite molar abundance | median | 9.4 mol% | highest | 48 mol% (oxygen) | lowest | 0.016 mol% (chlorine) ocean molar abundance | median | 17 mol% | highest | 66 mol% (hydrogen) | lowest | 0.0014 mol% (carbon) | distribution | crust molar abundance | median | 1.7 mol% | highest | 60 mol% (oxygen) | lowest | 0.0099 mol% (chlorine) human molar abundance | median | 18 mol% | highest | 62 mol% (hydrogen) | lowest | 0.021 mol% (chlorine) | distribution |