Input interpretation
antimony pentachloride
Chemical names and formulas
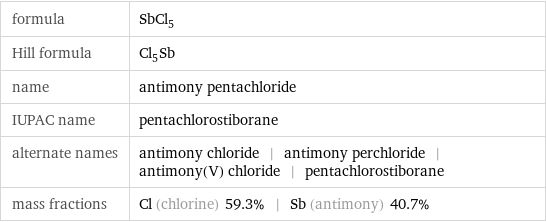
formula | SbCl_5 Hill formula | Cl_5Sb name | antimony pentachloride IUPAC name | pentachlorostiborane alternate names | antimony chloride | antimony perchloride | antimony(V) chloride | pentachlorostiborane mass fractions | Cl (chlorine) 59.3% | Sb (antimony) 40.7%
Lewis structure

Draw the Lewis structure of antimony pentachloride. Start by drawing the overall structure of the molecule: Count the total valence electrons of the chlorine (n_Cl, val = 7) and antimony (n_Sb, val = 5) atoms: 5 n_Cl, val + n_Sb, val = 40 Calculate the number of electrons needed to completely fill the valence shells for chlorine (n_Cl, full = 8) and antimony (n_Sb, full = 8): 5 n_Cl, full + n_Sb, full = 48 Subtracting these two numbers shows that 48 - 40 = 8 bonding electrons are needed, which are already accounted for in the structure. Note that the valence shell of antimony has been expanded to 5 bonds. After accounting for the expanded valence, there are 5 bonds and hence 10 bonding electrons in the diagram. Lastly, fill in the remaining unbonded electrons on each atom. In total, there remain 40 - 10 = 30 electrons left to draw: Answer: | |
Basic properties
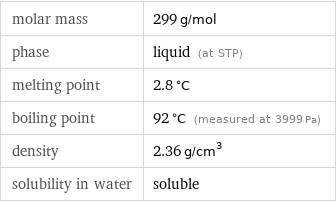
molar mass | 299 g/mol phase | liquid (at STP) melting point | 2.8 °C boiling point | 92 °C (measured at 3999 Pa) density | 2.36 g/cm^3 solubility in water | soluble
Units

Liquid properties (at STP)
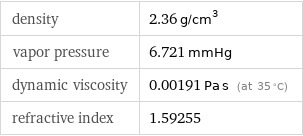
density | 2.36 g/cm^3 vapor pressure | 6.721 mmHg dynamic viscosity | 0.00191 Pa s (at 35 °C) refractive index | 1.59255
Units
Thermodynamic properties
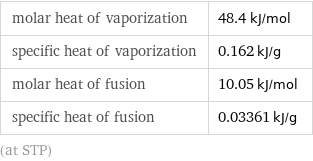
molar heat of vaporization | 48.4 kJ/mol specific heat of vaporization | 0.162 kJ/g molar heat of fusion | 10.05 kJ/mol specific heat of fusion | 0.03361 kJ/g (at STP)
Chemical identifiers
(Cl)(Cl)Cl InChI identifier | InChI=1/5ClH.Sb/h5*1H;/q;;;;;+5/p-5/f5Cl.Sb/h5*1h;/q5*-1;m/rCl5Sb/c1-6(2, 3, 4)5 RTECS number | CC5075000 MDL number | MFCD00011213](../image_source/e8eba878345838fb7aa3a9abafe02f67.png)
CAS number | 7647-18-9 PubChem CID number | 24294 PubChem SID number | 24852875 SMILES identifier | Cl[Sb](Cl)(Cl)(Cl)Cl InChI identifier | InChI=1/5ClH.Sb/h5*1H;/q;;;;;+5/p-5/f5Cl.Sb/h5*1h;/q5*-1;m/rCl5Sb/c1-6(2, 3, 4)5 RTECS number | CC5075000 MDL number | MFCD00011213
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 0 NFPA reactivity rating | 1
Safety properties

flash point | 77 °C

DOT hazard class | 8 DOT numbers | 1731
Toxicity properties

lethal dosage | 1150 mg/kg (oral dose for rats)

probable lethal dose for man | 600 mL (milliliters) RTECS classes | mutagen