Input interpretation
copper(II) nitrate | cobalt(II) carbonate hydrate
Chemical names and formulas
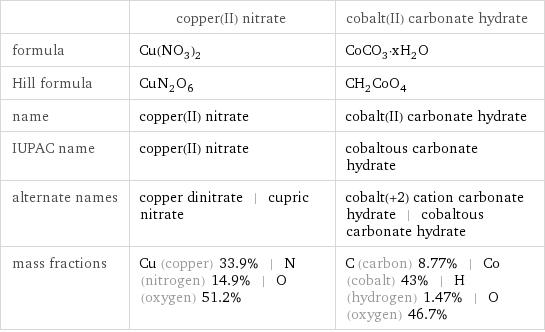
| copper(II) nitrate | cobalt(II) carbonate hydrate formula | Cu(NO_3)_2 | CoCO_3·xH_2O Hill formula | CuN_2O_6 | CH_2CoO_4 name | copper(II) nitrate | cobalt(II) carbonate hydrate IUPAC name | copper(II) nitrate | cobaltous carbonate hydrate alternate names | copper dinitrate | cupric nitrate | cobalt(+2) cation carbonate hydrate | cobaltous carbonate hydrate mass fractions | Cu (copper) 33.9% | N (nitrogen) 14.9% | O (oxygen) 51.2% | C (carbon) 8.77% | Co (cobalt) 43% | H (hydrogen) 1.47% | O (oxygen) 46.7%
Structure diagrams
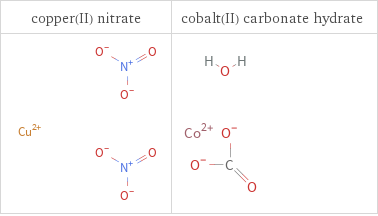
Structure diagrams
Basic properties
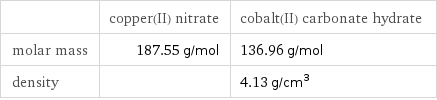
| copper(II) nitrate | cobalt(II) carbonate hydrate molar mass | 187.55 g/mol | 136.96 g/mol density | | 4.13 g/cm^3
Units
