Input interpretation
metalloids
Members
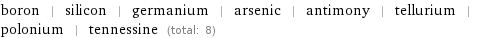
boron | silicon | germanium | arsenic | antimony | tellurium | polonium | tennessine (total: 8)
Periodic table location
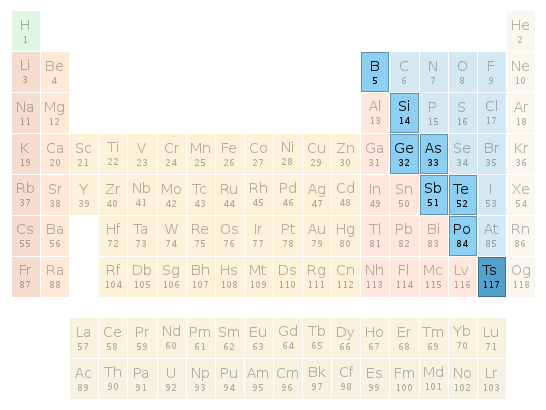
Periodic table location
Images
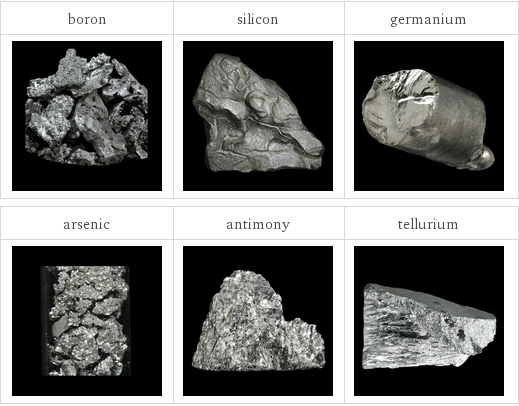
Images
Basic elemental properties
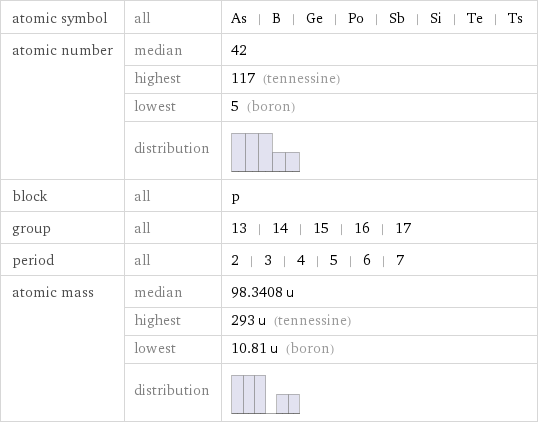
atomic symbol | all | As | B | Ge | Po | Sb | Si | Te | Ts atomic number | median | 42 | highest | 117 (tennessine) | lowest | 5 (boron) | distribution | block | all | p group | all | 13 | 14 | 15 | 16 | 17 period | all | 2 | 3 | 4 | 5 | 6 | 7 atomic mass | median | 98.3408 u | highest | 293 u (tennessine) | lowest | 10.81 u (boron) | distribution |
Thermodynamic properties
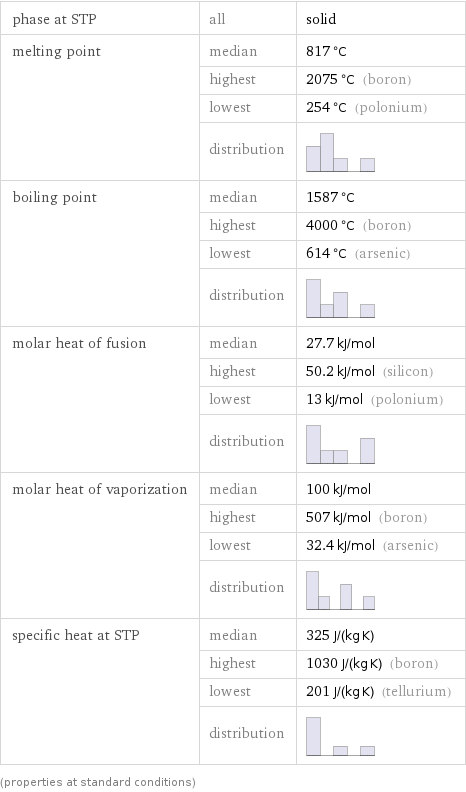
phase at STP | all | solid melting point | median | 817 °C | highest | 2075 °C (boron) | lowest | 254 °C (polonium) | distribution | boiling point | median | 1587 °C | highest | 4000 °C (boron) | lowest | 614 °C (arsenic) | distribution | molar heat of fusion | median | 27.7 kJ/mol | highest | 50.2 kJ/mol (silicon) | lowest | 13 kJ/mol (polonium) | distribution | molar heat of vaporization | median | 100 kJ/mol | highest | 507 kJ/mol (boron) | lowest | 32.4 kJ/mol (arsenic) | distribution | specific heat at STP | median | 325 J/(kg K) | highest | 1030 J/(kg K) (boron) | lowest | 201 J/(kg K) (tellurium) | distribution | (properties at standard conditions)
Material properties
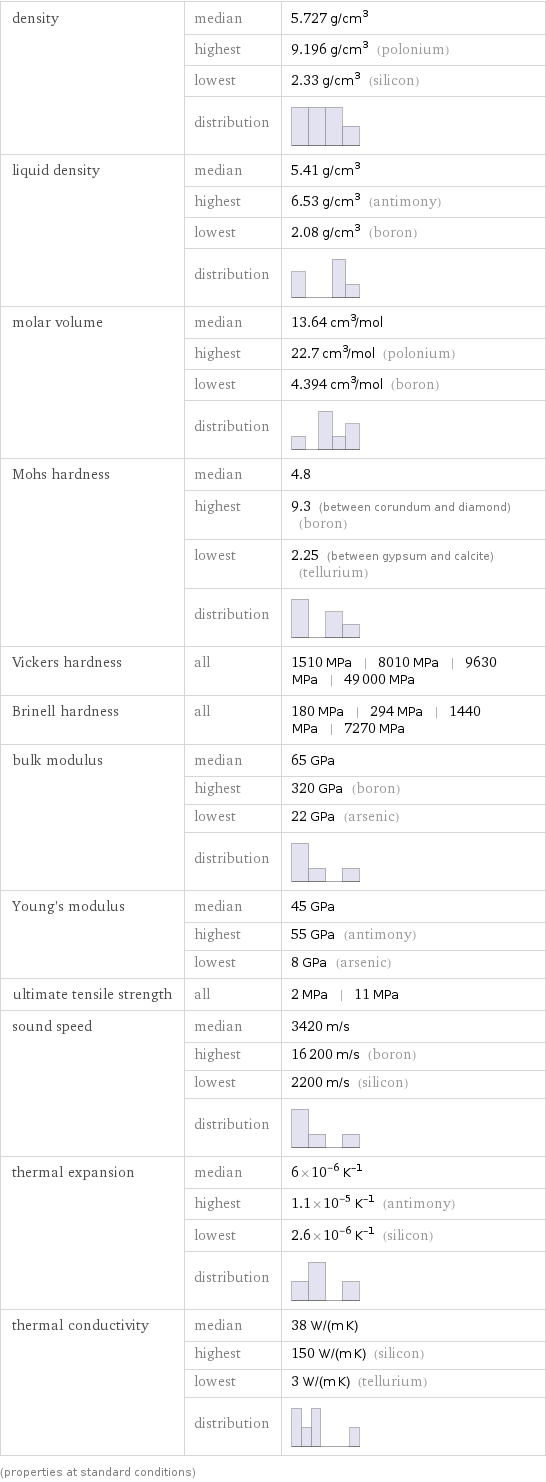
density | median | 5.727 g/cm^3 | highest | 9.196 g/cm^3 (polonium) | lowest | 2.33 g/cm^3 (silicon) | distribution | liquid density | median | 5.41 g/cm^3 | highest | 6.53 g/cm^3 (antimony) | lowest | 2.08 g/cm^3 (boron) | distribution | molar volume | median | 13.64 cm^3/mol | highest | 22.7 cm^3/mol (polonium) | lowest | 4.394 cm^3/mol (boron) | distribution | Mohs hardness | median | 4.8 | highest | 9.3 (between corundum and diamond) (boron) | lowest | 2.25 (between gypsum and calcite) (tellurium) | distribution | Vickers hardness | all | 1510 MPa | 8010 MPa | 9630 MPa | 49000 MPa Brinell hardness | all | 180 MPa | 294 MPa | 1440 MPa | 7270 MPa bulk modulus | median | 65 GPa | highest | 320 GPa (boron) | lowest | 22 GPa (arsenic) | distribution | Young's modulus | median | 45 GPa | highest | 55 GPa (antimony) | lowest | 8 GPa (arsenic) ultimate tensile strength | all | 2 MPa | 11 MPa sound speed | median | 3420 m/s | highest | 16200 m/s (boron) | lowest | 2200 m/s (silicon) | distribution | thermal expansion | median | 6×10^-6 K^(-1) | highest | 1.1×10^-5 K^(-1) (antimony) | lowest | 2.6×10^-6 K^(-1) (silicon) | distribution | thermal conductivity | median | 38 W/(m K) | highest | 150 W/(m K) (silicon) | lowest | 3 W/(m K) (tellurium) | distribution | (properties at standard conditions)
Electromagnetic properties
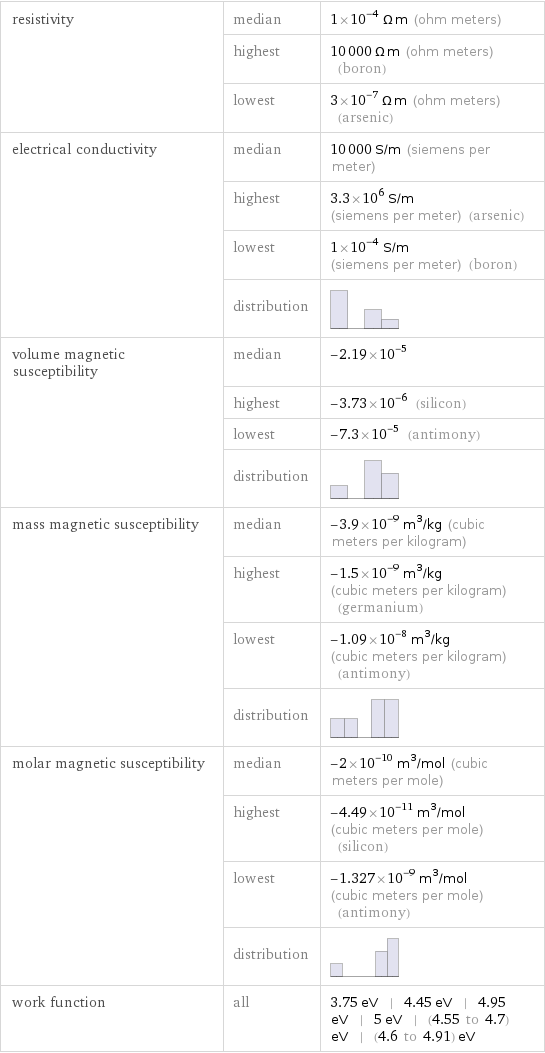
resistivity | median | 1×10^-4 Ω m (ohm meters) | highest | 10000 Ω m (ohm meters) (boron) | lowest | 3×10^-7 Ω m (ohm meters) (arsenic) electrical conductivity | median | 10000 S/m (siemens per meter) | highest | 3.3×10^6 S/m (siemens per meter) (arsenic) | lowest | 1×10^-4 S/m (siemens per meter) (boron) | distribution | volume magnetic susceptibility | median | -2.19×10^-5 | highest | -3.73×10^-6 (silicon) | lowest | -7.3×10^-5 (antimony) | distribution | mass magnetic susceptibility | median | -3.9×10^-9 m^3/kg (cubic meters per kilogram) | highest | -1.5×10^-9 m^3/kg (cubic meters per kilogram) (germanium) | lowest | -1.09×10^-8 m^3/kg (cubic meters per kilogram) (antimony) | distribution | molar magnetic susceptibility | median | -2×10^-10 m^3/mol (cubic meters per mole) | highest | -4.49×10^-11 m^3/mol (cubic meters per mole) (silicon) | lowest | -1.327×10^-9 m^3/mol (cubic meters per mole) (antimony) | distribution | work function | all | 3.75 eV | 4.45 eV | 4.95 eV | 5 eV | (4.55 to 4.7) eV | (4.6 to 4.91) eV
Reactivity
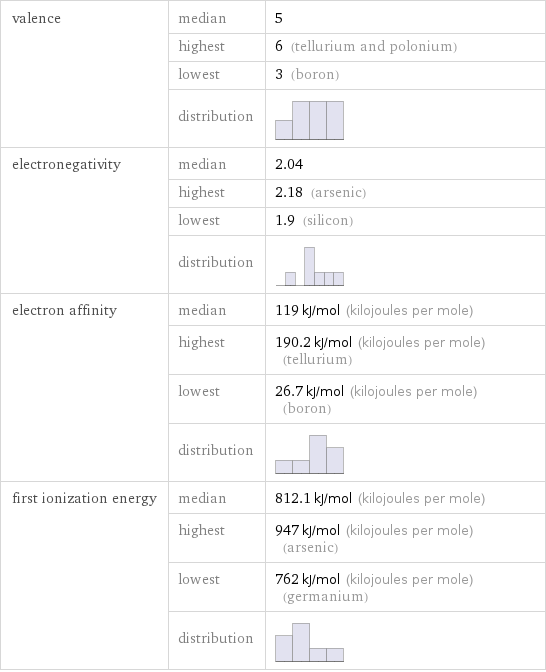
valence | median | 5 | highest | 6 (tellurium and polonium) | lowest | 3 (boron) | distribution | electronegativity | median | 2.04 | highest | 2.18 (arsenic) | lowest | 1.9 (silicon) | distribution | electron affinity | median | 119 kJ/mol (kilojoules per mole) | highest | 190.2 kJ/mol (kilojoules per mole) (tellurium) | lowest | 26.7 kJ/mol (kilojoules per mole) (boron) | distribution | first ionization energy | median | 812.1 kJ/mol (kilojoules per mole) | highest | 947 kJ/mol (kilojoules per mole) (arsenic) | lowest | 762 kJ/mol (kilojoules per mole) (germanium) | distribution |
Atomic properties
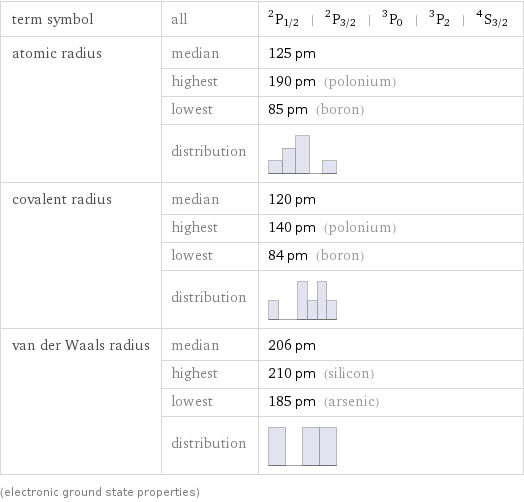
term symbol | all | ^2P_(1/2) | ^2P_(3/2) | ^3P_0 | ^3P_2 | ^4S_(3/2) atomic radius | median | 125 pm | highest | 190 pm (polonium) | lowest | 85 pm (boron) | distribution | covalent radius | median | 120 pm | highest | 140 pm (polonium) | lowest | 84 pm (boron) | distribution | van der Waals radius | median | 206 pm | highest | 210 pm (silicon) | lowest | 185 pm (arsenic) | distribution | (electronic ground state properties)