Input interpretation
rhodium (chemical element) | manganese (chemical element) | titanium (chemical element)
Periodic table location
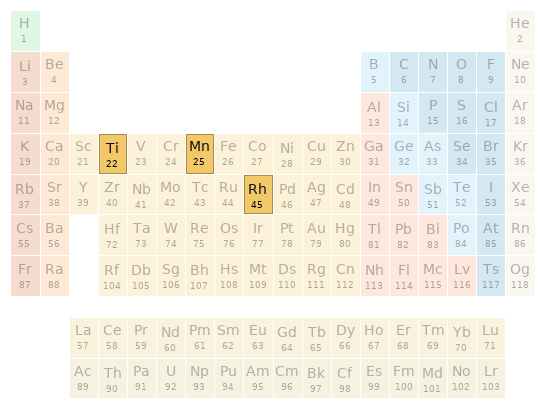
Periodic table location
Images

Images
Basic elemental properties
![| rhodium | manganese | titanium atomic symbol | Rh | Mn | Ti atomic number | 45 | 25 | 22 short electronic configuration | [Kr]5s^14d^8 | [Ar]4s^23d^5 | [Ar]4s^23d^2 Aufbau diagram | 4d 5s | 3d 4s | 3d 4s block | d | d | d group | 9 | 7 | 4 period | 5 | 4 | 4 atomic mass | 102.9055 u | 54.938044 u | 47.867 u half-life | (stable) | (stable) | (stable)](../image_source/c865d1608d8a566d6db93e09bd7e8f56.png)
| rhodium | manganese | titanium atomic symbol | Rh | Mn | Ti atomic number | 45 | 25 | 22 short electronic configuration | [Kr]5s^14d^8 | [Ar]4s^23d^5 | [Ar]4s^23d^2 Aufbau diagram | 4d 5s | 3d 4s | 3d 4s block | d | d | d group | 9 | 7 | 4 period | 5 | 4 | 4 atomic mass | 102.9055 u | 54.938044 u | 47.867 u half-life | (stable) | (stable) | (stable)
Thermodynamic properties
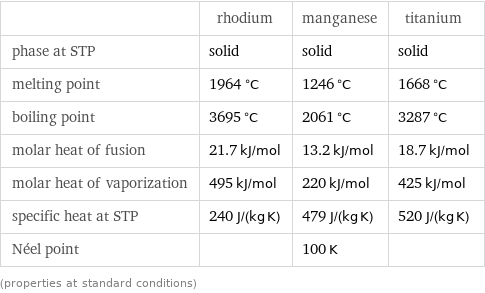
| rhodium | manganese | titanium phase at STP | solid | solid | solid melting point | 1964 °C | 1246 °C | 1668 °C boiling point | 3695 °C | 2061 °C | 3287 °C molar heat of fusion | 21.7 kJ/mol | 13.2 kJ/mol | 18.7 kJ/mol molar heat of vaporization | 495 kJ/mol | 220 kJ/mol | 425 kJ/mol specific heat at STP | 240 J/(kg K) | 479 J/(kg K) | 520 J/(kg K) Néel point | | 100 K | (properties at standard conditions)
Material properties
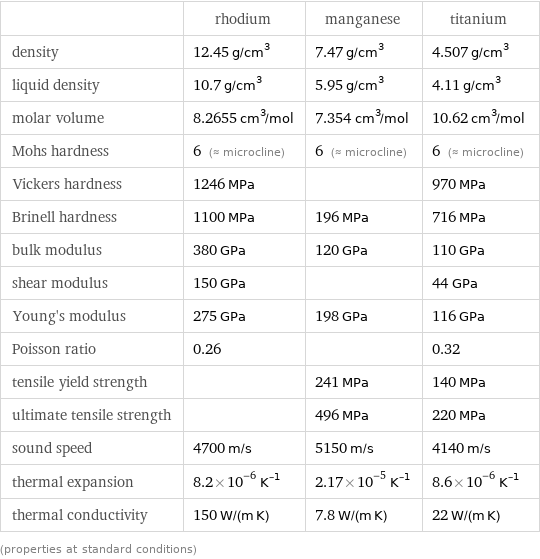
| rhodium | manganese | titanium density | 12.45 g/cm^3 | 7.47 g/cm^3 | 4.507 g/cm^3 liquid density | 10.7 g/cm^3 | 5.95 g/cm^3 | 4.11 g/cm^3 molar volume | 8.2655 cm^3/mol | 7.354 cm^3/mol | 10.62 cm^3/mol Mohs hardness | 6 (≈ microcline) | 6 (≈ microcline) | 6 (≈ microcline) Vickers hardness | 1246 MPa | | 970 MPa Brinell hardness | 1100 MPa | 196 MPa | 716 MPa bulk modulus | 380 GPa | 120 GPa | 110 GPa shear modulus | 150 GPa | | 44 GPa Young's modulus | 275 GPa | 198 GPa | 116 GPa Poisson ratio | 0.26 | | 0.32 tensile yield strength | | 241 MPa | 140 MPa ultimate tensile strength | | 496 MPa | 220 MPa sound speed | 4700 m/s | 5150 m/s | 4140 m/s thermal expansion | 8.2×10^-6 K^(-1) | 2.17×10^-5 K^(-1) | 8.6×10^-6 K^(-1) thermal conductivity | 150 W/(m K) | 7.8 W/(m K) | 22 W/(m K) (properties at standard conditions)
Electromagnetic properties
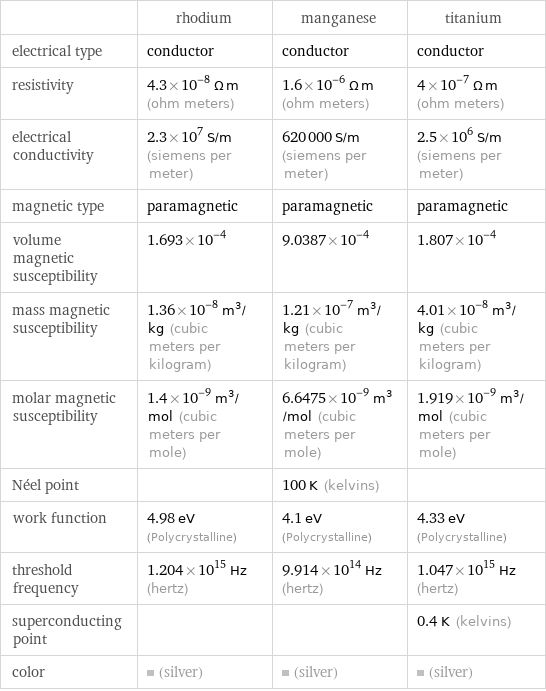
| rhodium | manganese | titanium electrical type | conductor | conductor | conductor resistivity | 4.3×10^-8 Ω m (ohm meters) | 1.6×10^-6 Ω m (ohm meters) | 4×10^-7 Ω m (ohm meters) electrical conductivity | 2.3×10^7 S/m (siemens per meter) | 620000 S/m (siemens per meter) | 2.5×10^6 S/m (siemens per meter) magnetic type | paramagnetic | paramagnetic | paramagnetic volume magnetic susceptibility | 1.693×10^-4 | 9.0387×10^-4 | 1.807×10^-4 mass magnetic susceptibility | 1.36×10^-8 m^3/kg (cubic meters per kilogram) | 1.21×10^-7 m^3/kg (cubic meters per kilogram) | 4.01×10^-8 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | 1.4×10^-9 m^3/mol (cubic meters per mole) | 6.6475×10^-9 m^3/mol (cubic meters per mole) | 1.919×10^-9 m^3/mol (cubic meters per mole) Néel point | | 100 K (kelvins) | work function | 4.98 eV (Polycrystalline) | 4.1 eV (Polycrystalline) | 4.33 eV (Polycrystalline) threshold frequency | 1.204×10^15 Hz (hertz) | 9.914×10^14 Hz (hertz) | 1.047×10^15 Hz (hertz) superconducting point | | | 0.4 K (kelvins) color | (silver) | (silver) | (silver)
Reactivity

| rhodium | manganese | titanium valence | 6 | 4 | 4 electronegativity | 2.28 | 1.55 | 1.54 electron affinity | 109.7 kJ/mol (kilojoules per mole) | 0 kJ/mol (kilojoules per mole) | 7.6 kJ/mol (kilojoules per mole) first ionization energy | 719.7 kJ/mol (kilojoules per mole) | 717.3 kJ/mol (kilojoules per mole) | 658.8 kJ/mol (kilojoules per mole) ionization energies | 719.7 kJ/mol | 1740 kJ/mol | 2997 kJ/mol | 717.3 kJ/mol | 1509 kJ/mol | 3248 kJ/mol | 4940 kJ/mol | 6990 kJ/mol | 9220 kJ/mol | 11500 kJ/mol | 18770 kJ/mol | 21400 kJ/mol | 23960 kJ/mol | 27590 kJ/mol | 30330 kJ/mol | 33150 kJ/mol | 38880 kJ/mol | 41987 kJ/mol | 109480 kJ/mol | 118100 kJ/mol | 127100 kJ/mol | 138600 kJ/mol | 148500 kJ/mol | 158600 kJ/mol | 658.8 kJ/mol | 1309.8 kJ/mol | 2652.5 kJ/mol | 4174.6 kJ/mol | 9581 kJ/mol | 11533 kJ/mol | 13590 kJ/mol | 16440 kJ/mol | 18530 kJ/mol | 20833 kJ/mol | 25575 kJ/mol | 28125 kJ/mol | 76015 kJ/mol | 83280 kJ/mol | 90880 kJ/mol | 100700 kJ/mol | 109100 kJ/mol | 117800 kJ/mol | 129900 kJ/mol | 137530 kJ/mol | 602930 kJ/mol
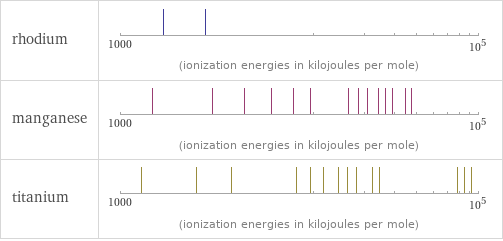
Reactivity
Atomic properties
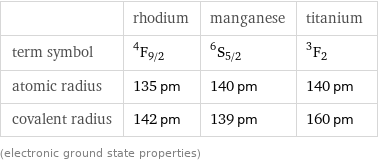
| rhodium | manganese | titanium term symbol | ^4F_(9/2) | ^6S_(5/2) | ^3F_2 atomic radius | 135 pm | 140 pm | 140 pm covalent radius | 142 pm | 139 pm | 160 pm (electronic ground state properties)
Abundances
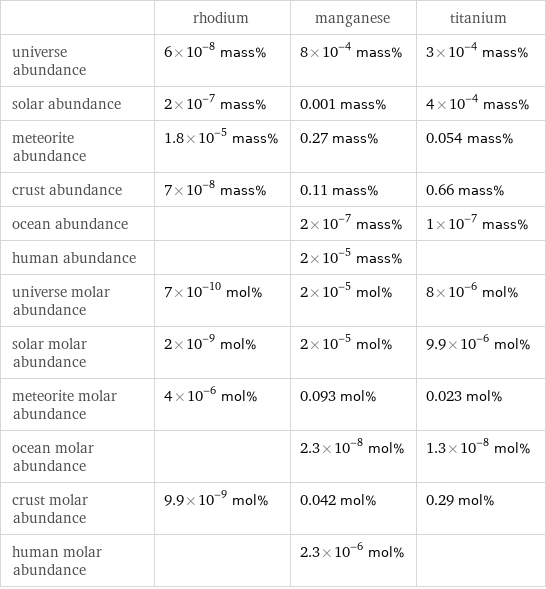
| rhodium | manganese | titanium universe abundance | 6×10^-8 mass% | 8×10^-4 mass% | 3×10^-4 mass% solar abundance | 2×10^-7 mass% | 0.001 mass% | 4×10^-4 mass% meteorite abundance | 1.8×10^-5 mass% | 0.27 mass% | 0.054 mass% crust abundance | 7×10^-8 mass% | 0.11 mass% | 0.66 mass% ocean abundance | | 2×10^-7 mass% | 1×10^-7 mass% human abundance | | 2×10^-5 mass% | universe molar abundance | 7×10^-10 mol% | 2×10^-5 mol% | 8×10^-6 mol% solar molar abundance | 2×10^-9 mol% | 2×10^-5 mol% | 9.9×10^-6 mol% meteorite molar abundance | 4×10^-6 mol% | 0.093 mol% | 0.023 mol% ocean molar abundance | | 2.3×10^-8 mol% | 1.3×10^-8 mol% crust molar abundance | 9.9×10^-9 mol% | 0.042 mol% | 0.29 mol% human molar abundance | | 2.3×10^-6 mol% |