Input interpretation
ammonium tetrafluoroantimonate | zirconium tetrachloride
Chemical names and formulas
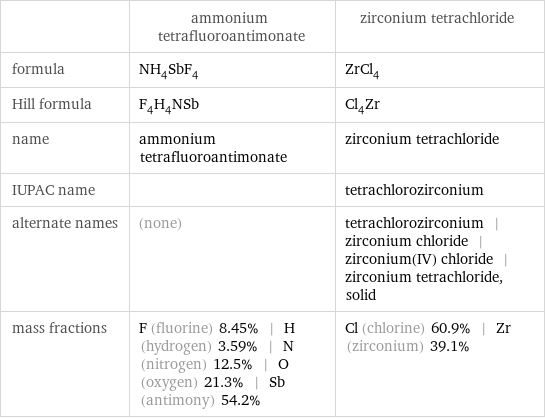
| ammonium tetrafluoroantimonate | zirconium tetrachloride formula | NH_4SbF_4 | ZrCl_4 Hill formula | F_4H_4NSb | Cl_4Zr name | ammonium tetrafluoroantimonate | zirconium tetrachloride IUPAC name | | tetrachlorozirconium alternate names | (none) | tetrachlorozirconium | zirconium chloride | zirconium(IV) chloride | zirconium tetrachloride, solid mass fractions | F (fluorine) 8.45% | H (hydrogen) 3.59% | N (nitrogen) 12.5% | O (oxygen) 21.3% | Sb (antimony) 54.2% | Cl (chlorine) 60.9% | Zr (zirconium) 39.1%
Structure diagrams
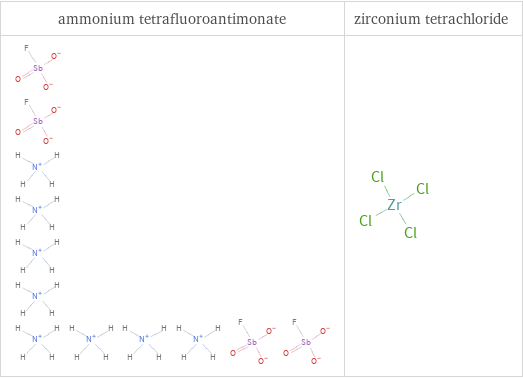
Structure diagrams
Basic properties
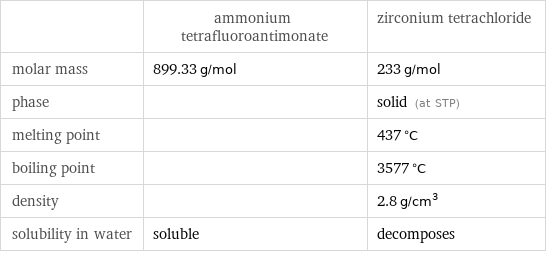
| ammonium tetrafluoroantimonate | zirconium tetrachloride molar mass | 899.33 g/mol | 233 g/mol phase | | solid (at STP) melting point | | 437 °C boiling point | | 3577 °C density | | 2.8 g/cm^3 solubility in water | soluble | decomposes
Units

Thermodynamic properties
| zirconium tetrachloride specific heat of vaporization | 0.4536 kJ/g (kilojoules per gram) critical temperature | 776.6 K (kelvins) critical pressure | 5.7 MPa (megapascals)