Input interpretation
arsenic(III) sulfide | aluminum sulfate
Chemical names and formulas
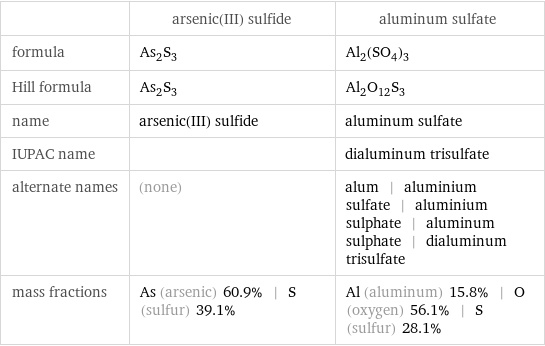
| arsenic(III) sulfide | aluminum sulfate formula | As_2S_3 | Al_2(SO_4)_3 Hill formula | As_2S_3 | Al_2O_12S_3 name | arsenic(III) sulfide | aluminum sulfate IUPAC name | | dialuminum trisulfate alternate names | (none) | alum | aluminium sulfate | aluminium sulphate | aluminum sulphate | dialuminum trisulfate mass fractions | As (arsenic) 60.9% | S (sulfur) 39.1% | Al (aluminum) 15.8% | O (oxygen) 56.1% | S (sulfur) 28.1%
Structure diagrams
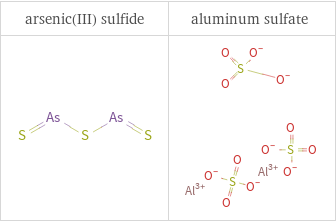
Structure diagrams
Basic properties
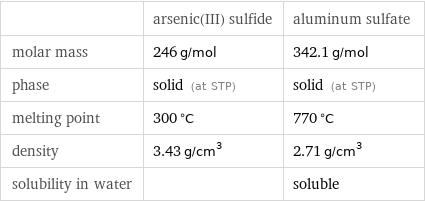
| arsenic(III) sulfide | aluminum sulfate molar mass | 246 g/mol | 342.1 g/mol phase | solid (at STP) | solid (at STP) melting point | 300 °C | 770 °C density | 3.43 g/cm^3 | 2.71 g/cm^3 solubility in water | | soluble
Units

Solid properties (at STP)

| arsenic(III) sulfide | aluminum sulfate density | 3.43 g/cm^3 | 2.71 g/cm^3 vapor pressure | | 120 mmHg
Units
