Input interpretation

dying low mass star elements | first molar ionization energy
Summary
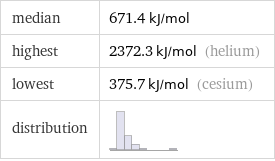
median | 671.4 kJ/mol highest | 2372.3 kJ/mol (helium) lowest | 375.7 kJ/mol (cesium) distribution |
Units

Distribution plots
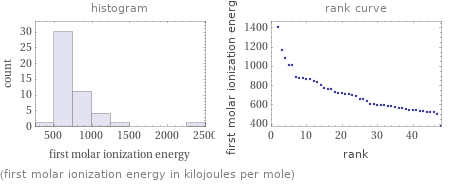
(first molar ionization energy in kilojoules per mole)
First molar ionization energy rankings
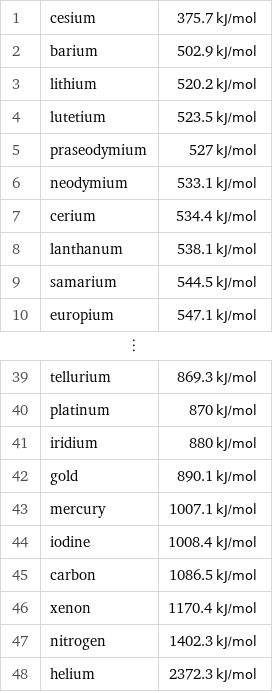
1 | cesium | 375.7 kJ/mol 2 | barium | 502.9 kJ/mol 3 | lithium | 520.2 kJ/mol 4 | lutetium | 523.5 kJ/mol 5 | praseodymium | 527 kJ/mol 6 | neodymium | 533.1 kJ/mol 7 | cerium | 534.4 kJ/mol 8 | lanthanum | 538.1 kJ/mol 9 | samarium | 544.5 kJ/mol 10 | europium | 547.1 kJ/mol ⋮ | | 39 | tellurium | 869.3 kJ/mol 40 | platinum | 870 kJ/mol 41 | iridium | 880 kJ/mol 42 | gold | 890.1 kJ/mol 43 | mercury | 1007.1 kJ/mol 44 | iodine | 1008.4 kJ/mol 45 | carbon | 1086.5 kJ/mol 46 | xenon | 1170.4 kJ/mol 47 | nitrogen | 1402.3 kJ/mol 48 | helium | 2372.3 kJ/mol
Unit conversions for median first molar ionization energy 671.4 kJ/mol

0.6714 MJ/mol (megajoules per mole)
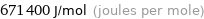
671400 J/mol (joules per mole)
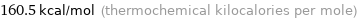
160.5 kcal/mol (thermochemical kilocalories per mole)

6.959 eV (molar electronvolts)
Comparisons for median first molar ionization energy 671.4 kJ/mol

≈ 0.28 × molar energy required to remove the first electron from helium ( 2372 kJ/mol )
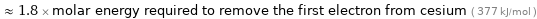
≈ 1.8 × molar energy required to remove the first electron from cesium ( 377 kJ/mol )