Input interpretation
copper(I) sulfide
Chemical names and formulas

formula | Cu_2S Hill formula | Cu_2S_1 name | copper(I) sulfide alternate names | copper; copper(+1) cation; sulfanide | cuprous copper bisulfide | cuprous; copper; sulfanide mass fractions | Cu (copper) 66.5% | S (sulfur) 33.5%
Structure diagram
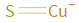
Structure diagram
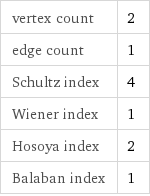
vertex count | 2 edge count | 1 Schultz index | 4 Wiener index | 1 Hosoya index | 2 Balaban index | 1
Basic properties
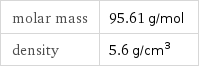
molar mass | 95.61 g/mol density | 5.6 g/cm^3
Units

Thermodynamic properties
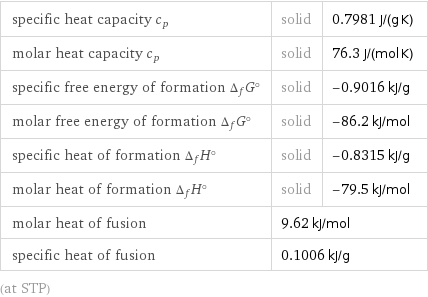
specific heat capacity c_p | solid | 0.7981 J/(g K) molar heat capacity c_p | solid | 76.3 J/(mol K) specific free energy of formation Δ_fG° | solid | -0.9016 kJ/g molar free energy of formation Δ_fG° | solid | -86.2 kJ/mol specific heat of formation Δ_fH° | solid | -0.8315 kJ/g molar heat of formation Δ_fH° | solid | -79.5 kJ/mol molar heat of fusion | 9.62 kJ/mol | specific heat of fusion | 0.1006 kJ/g | (at STP)
Chemical identifiers
![CAS number | 22205-45-4 SMILES identifier | [Cu-]=S RTECS number | GL8910000 MDL number | MFCD00016062](../image_source/725cf115271b329213d42bd23d633932.png)
CAS number | 22205-45-4 SMILES identifier | [Cu-]=S RTECS number | GL8910000 MDL number | MFCD00016062