Input interpretation
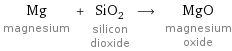
Mg magnesium + SiO_2 silicon dioxide ⟶ MgO magnesium oxide
Chemical names and formulas
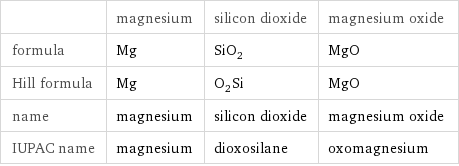
| magnesium | silicon dioxide | magnesium oxide formula | Mg | SiO_2 | MgO Hill formula | Mg | O_2Si | MgO name | magnesium | silicon dioxide | magnesium oxide IUPAC name | magnesium | dioxosilane | oxomagnesium
Substance properties
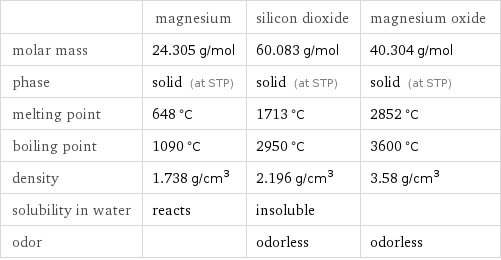
| magnesium | silicon dioxide | magnesium oxide molar mass | 24.305 g/mol | 60.083 g/mol | 40.304 g/mol phase | solid (at STP) | solid (at STP) | solid (at STP) melting point | 648 °C | 1713 °C | 2852 °C boiling point | 1090 °C | 2950 °C | 3600 °C density | 1.738 g/cm^3 | 2.196 g/cm^3 | 3.58 g/cm^3 solubility in water | reacts | insoluble | odor | | odorless | odorless
Units
