Input interpretation
dilithium tetrabromocuprate(II) | magnesium antimonide
Chemical names and formulas
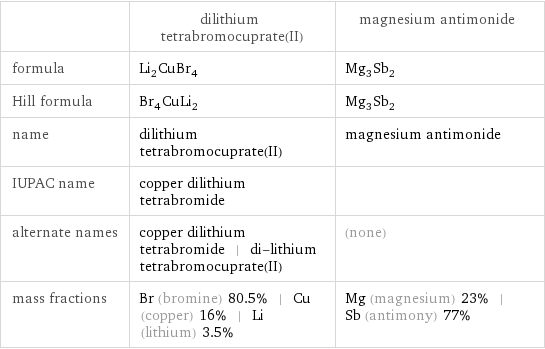
| dilithium tetrabromocuprate(II) | magnesium antimonide formula | Li_2CuBr_4 | Mg_3Sb_2 Hill formula | Br_4CuLi_2 | Mg_3Sb_2 name | dilithium tetrabromocuprate(II) | magnesium antimonide IUPAC name | copper dilithium tetrabromide | alternate names | copper dilithium tetrabromide | di-lithium tetrabromocuprate(II) | (none) mass fractions | Br (bromine) 80.5% | Cu (copper) 16% | Li (lithium) 3.5% | Mg (magnesium) 23% | Sb (antimony) 77%
Structure diagrams
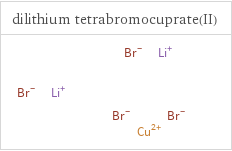
Structure diagrams
Basic properties
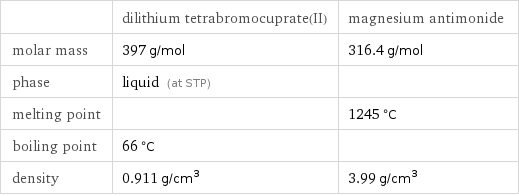
| dilithium tetrabromocuprate(II) | magnesium antimonide molar mass | 397 g/mol | 316.4 g/mol phase | liquid (at STP) | melting point | | 1245 °C boiling point | 66 °C | density | 0.911 g/cm^3 | 3.99 g/cm^3
Units
