Input interpretation
sodium periodate | chromium nitrate
Chemical names and formulas
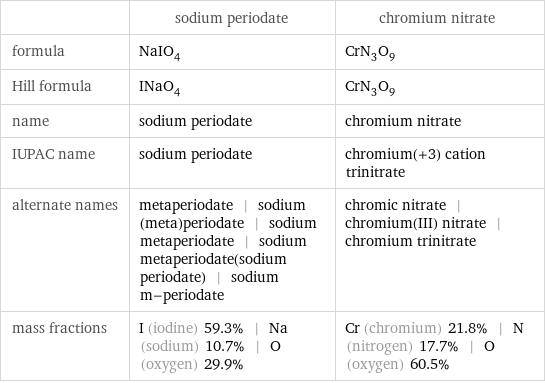
| sodium periodate | chromium nitrate formula | NaIO_4 | CrN_3O_9 Hill formula | INaO_4 | CrN_3O_9 name | sodium periodate | chromium nitrate IUPAC name | sodium periodate | chromium(+3) cation trinitrate alternate names | metaperiodate | sodium (meta)periodate | sodium metaperiodate | sodium metaperiodate(sodium periodate) | sodium m-periodate | chromic nitrate | chromium(III) nitrate | chromium trinitrate mass fractions | I (iodine) 59.3% | Na (sodium) 10.7% | O (oxygen) 29.9% | Cr (chromium) 21.8% | N (nitrogen) 17.7% | O (oxygen) 60.5%
Structure diagrams
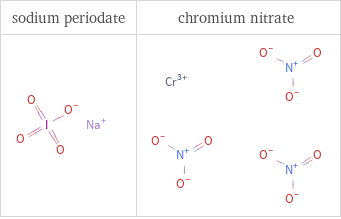
Structure diagrams
Basic properties
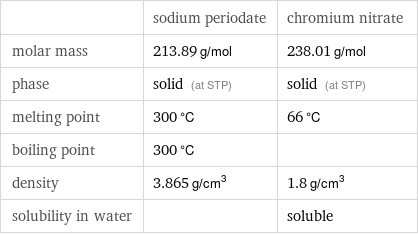
| sodium periodate | chromium nitrate molar mass | 213.89 g/mol | 238.01 g/mol phase | solid (at STP) | solid (at STP) melting point | 300 °C | 66 °C boiling point | 300 °C | density | 3.865 g/cm^3 | 1.8 g/cm^3 solubility in water | | soluble
Units
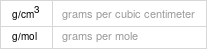
Solid properties (at STP)

| sodium periodate | chromium nitrate density | 3.865 g/cm^3 | 1.8 g/cm^3
Units
