Input interpretation
mercury
Chemical names and formulas

formula | Hg name | mercury alternate names | hydrargyrum | mercury metal | quicksilver mass fractions | Hg (mercury) 100%
Structure diagram

Structure diagram
Basic properties
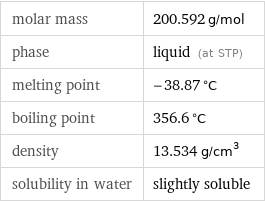
molar mass | 200.592 g/mol phase | liquid (at STP) melting point | -38.87 °C boiling point | 356.6 °C density | 13.534 g/cm^3 solubility in water | slightly soluble
Units

Liquid properties (at STP)
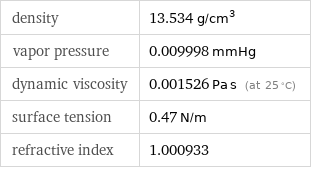
density | 13.534 g/cm^3 vapor pressure | 0.009998 mmHg dynamic viscosity | 0.001526 Pa s (at 25 °C) surface tension | 0.47 N/m refractive index | 1.000933
Units
Thermodynamic properties
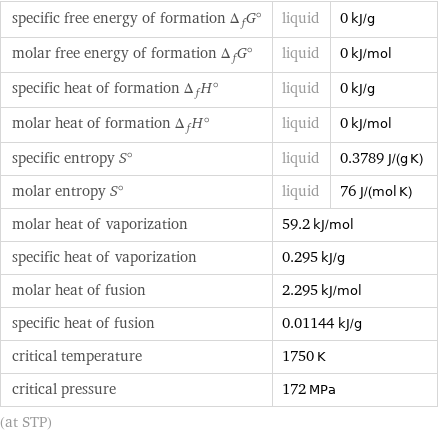
specific free energy of formation Δ_fG° | liquid | 0 kJ/g molar free energy of formation Δ_fG° | liquid | 0 kJ/mol specific heat of formation Δ_fH° | liquid | 0 kJ/g molar heat of formation Δ_fH° | liquid | 0 kJ/mol specific entropy S° | liquid | 0.3789 J/(g K) molar entropy S° | liquid | 76 J/(mol K) molar heat of vaporization | 59.2 kJ/mol | specific heat of vaporization | 0.295 kJ/g | molar heat of fusion | 2.295 kJ/mol | specific heat of fusion | 0.01144 kJ/g | critical temperature | 1750 K | critical pressure | 172 MPa | (at STP)
Chemical identifiers
![CAS number | 7439-97-6 PubChem CID number | 23931 PubChem SID number | 24855784 SMILES identifier | [Hg] InChI identifier | InChI=1/Hg RTECS number | OV4550000 MDL number | MFCD00011035](../image_source/733c28be262325e9bbb41aeffb25454f.png)
CAS number | 7439-97-6 PubChem CID number | 23931 PubChem SID number | 24855784 SMILES identifier | [Hg] InChI identifier | InChI=1/Hg RTECS number | OV4550000 MDL number | MFCD00011035
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 0 NFPA reactivity rating | 0
Toxicity properties

odor | odorless

RTECS classes | agricultural chemical and pesticide | tumorigen | drug | mutagen | reproductive effector | human data