Input interpretation
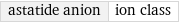
astatide anion | ion class
Result
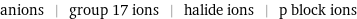
anions | group 17 ions | halide ions | p block ions
Ionic radii
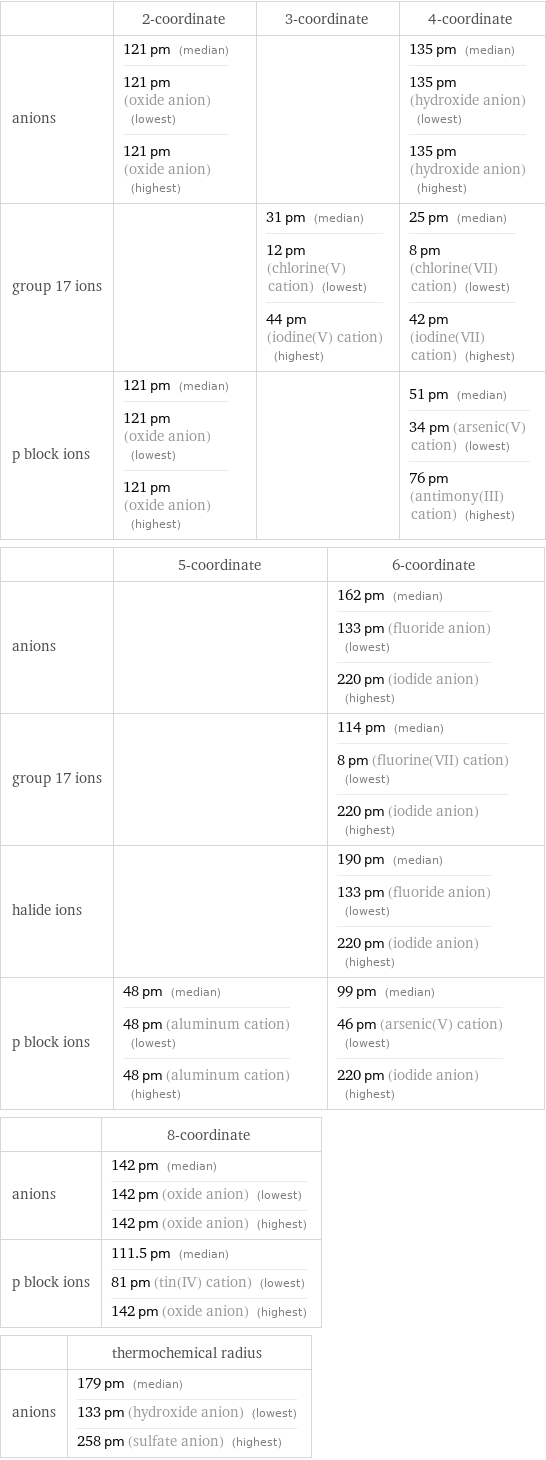
| 2-coordinate | 3-coordinate | 4-coordinate anions | 121 pm (median) 121 pm (oxide anion) (lowest) 121 pm (oxide anion) (highest) | | 135 pm (median) 135 pm (hydroxide anion) (lowest) 135 pm (hydroxide anion) (highest) group 17 ions | | 31 pm (median) 12 pm (chlorine(V) cation) (lowest) 44 pm (iodine(V) cation) (highest) | 25 pm (median) 8 pm (chlorine(VII) cation) (lowest) 42 pm (iodine(VII) cation) (highest) p block ions | 121 pm (median) 121 pm (oxide anion) (lowest) 121 pm (oxide anion) (highest) | | 51 pm (median) 34 pm (arsenic(V) cation) (lowest) 76 pm (antimony(III) cation) (highest) | 5-coordinate | 6-coordinate anions | | 162 pm (median) 133 pm (fluoride anion) (lowest) 220 pm (iodide anion) (highest) group 17 ions | | 114 pm (median) 8 pm (fluorine(VII) cation) (lowest) 220 pm (iodide anion) (highest) halide ions | | 190 pm (median) 133 pm (fluoride anion) (lowest) 220 pm (iodide anion) (highest) p block ions | 48 pm (median) 48 pm (aluminum cation) (lowest) 48 pm (aluminum cation) (highest) | 99 pm (median) 46 pm (arsenic(V) cation) (lowest) 220 pm (iodide anion) (highest) | 8-coordinate anions | 142 pm (median) 142 pm (oxide anion) (lowest) 142 pm (oxide anion) (highest) p block ions | 111.5 pm (median) 81 pm (tin(IV) cation) (lowest) 142 pm (oxide anion) (highest) | thermochemical radius anions | 179 pm (median) 133 pm (hydroxide anion) (lowest) 258 pm (sulfate anion) (highest)