Input interpretation

period 6 elements | number of electrons
Summary
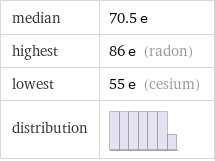
median | 70.5 e highest | 86 e (radon) lowest | 55 e (cesium) distribution |
Units

Distribution plots
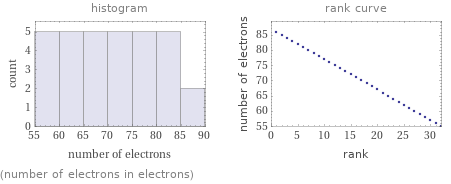
(number of electrons in electrons)
Number of electrons rankings
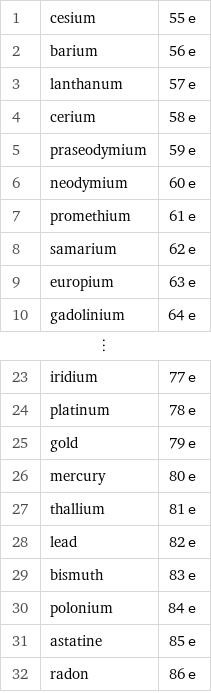
1 | cesium | 55 e 2 | barium | 56 e 3 | lanthanum | 57 e 4 | cerium | 58 e 5 | praseodymium | 59 e 6 | neodymium | 60 e 7 | promethium | 61 e 8 | samarium | 62 e 9 | europium | 63 e 10 | gadolinium | 64 e ⋮ | | 23 | iridium | 77 e 24 | platinum | 78 e 25 | gold | 79 e 26 | mercury | 80 e 27 | thallium | 81 e 28 | lead | 82 e 29 | bismuth | 83 e 30 | polonium | 84 e 31 | astatine | 85 e 32 | radon | 86 e
Corresponding quantity
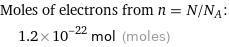
Moles of electrons from n = N/N_A: | 1.2×10^-22 mol (moles)