Input interpretation

non-metallic elements | electron affinity
Summary
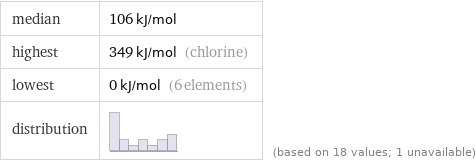
median | 106 kJ/mol highest | 349 kJ/mol (chlorine) lowest | 0 kJ/mol (6 elements) distribution | | (based on 18 values; 1 unavailable)
Entity with missing value
oganesson
Plot
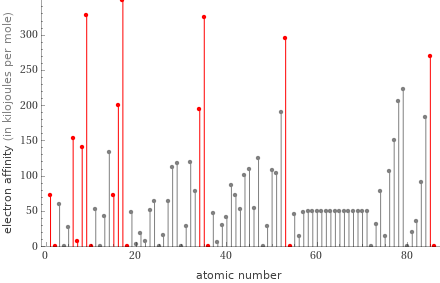
Plot
Periodic table location
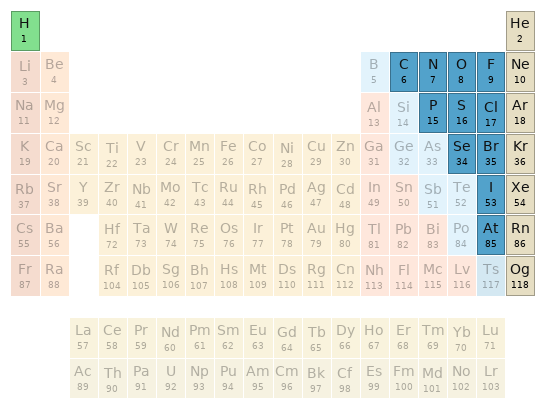
Periodic table location
Distribution plots
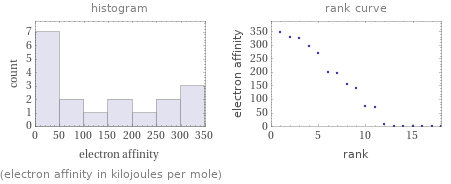
(electron affinity in kilojoules per mole)
Electron affinity rankings
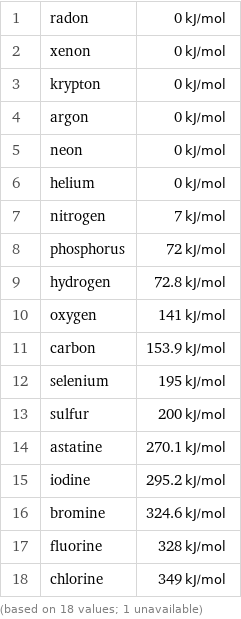
1 | radon | 0 kJ/mol 2 | xenon | 0 kJ/mol 3 | krypton | 0 kJ/mol 4 | argon | 0 kJ/mol 5 | neon | 0 kJ/mol 6 | helium | 0 kJ/mol 7 | nitrogen | 7 kJ/mol 8 | phosphorus | 72 kJ/mol 9 | hydrogen | 72.8 kJ/mol 10 | oxygen | 141 kJ/mol 11 | carbon | 153.9 kJ/mol 12 | selenium | 195 kJ/mol 13 | sulfur | 200 kJ/mol 14 | astatine | 270.1 kJ/mol 15 | iodine | 295.2 kJ/mol 16 | bromine | 324.6 kJ/mol 17 | fluorine | 328 kJ/mol 18 | chlorine | 349 kJ/mol (based on 18 values; 1 unavailable)
Unit conversions for median electron affinity 106 kJ/mol
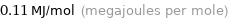
0.11 MJ/mol (megajoules per mole)
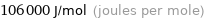
106000 J/mol (joules per mole)
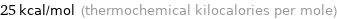
25 kcal/mol (thermochemical kilocalories per mole)
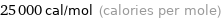
25000 cal/mol (calories per mole)
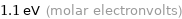
1.1 eV (molar electronvolts)