Input interpretation

O_2 oxygen + H_2S hydrogen sulfide ⟶ H_2SO_4 sulfuric acid
Balanced equation
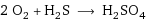
2 O_2 + H_2S ⟶ H_2SO_4
Structures

+ ⟶
Names
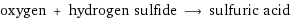
oxygen + hydrogen sulfide ⟶ sulfuric acid
Reaction thermodynamics
Enthalpy

ΔH_rxn^0 | -814 kJ/mol - -20.6 kJ/mol = -793.4 kJ/mol (exothermic)
Gibbs free energy

ΔG_rxn^0 | -690 kJ/mol - 430 kJ/mol = -1120 kJ/mol (exergonic)
Entropy

ΔS_rxn^0 | 157 J/(mol K) - 616 J/(mol K) = -459 J/(mol K) (exoentropic)
Units

Equilibrium constant
![K_c = [H2SO4]/([O2]^2 [H2S])](../image_source/e6552b992d9f8c16f5eb9ef1d734b0a4.png)
K_c = [H2SO4]/([O2]^2 [H2S])
Rate of reaction
![rate = -1/2 (Δ[O2])/(Δt) = -(Δ[H2S])/(Δt) = (Δ[H2SO4])/(Δt) (assuming constant volume and no accumulation of intermediates or side products)](../image_source/80461db27929c49431516d0a9cfe0420.png)
rate = -1/2 (Δ[O2])/(Δt) = -(Δ[H2S])/(Δt) = (Δ[H2SO4])/(Δt) (assuming constant volume and no accumulation of intermediates or side products)
Chemical names and formulas
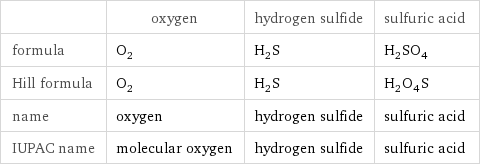
| oxygen | hydrogen sulfide | sulfuric acid formula | O_2 | H_2S | H_2SO_4 Hill formula | O_2 | H_2S | H_2O_4S name | oxygen | hydrogen sulfide | sulfuric acid IUPAC name | molecular oxygen | hydrogen sulfide | sulfuric acid
Substance properties
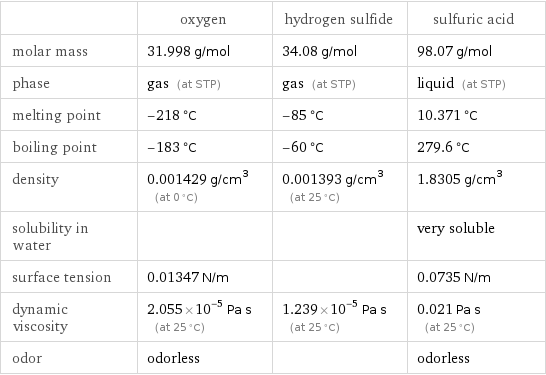
| oxygen | hydrogen sulfide | sulfuric acid molar mass | 31.998 g/mol | 34.08 g/mol | 98.07 g/mol phase | gas (at STP) | gas (at STP) | liquid (at STP) melting point | -218 °C | -85 °C | 10.371 °C boiling point | -183 °C | -60 °C | 279.6 °C density | 0.001429 g/cm^3 (at 0 °C) | 0.001393 g/cm^3 (at 25 °C) | 1.8305 g/cm^3 solubility in water | | | very soluble surface tension | 0.01347 N/m | | 0.0735 N/m dynamic viscosity | 2.055×10^-5 Pa s (at 25 °C) | 1.239×10^-5 Pa s (at 25 °C) | 0.021 Pa s (at 25 °C) odor | odorless | | odorless
Units
