Input interpretation
thionyl fluoride
Basic properties
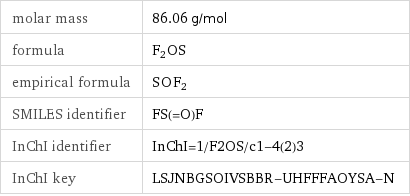
molar mass | 86.06 g/mol formula | F_2OS empirical formula | S_O_F_2 SMILES identifier | FS(=O)F InChI identifier | InChI=1/F2OS/c1-4(2)3 InChI key | LSJNBGSOIVSBBR-UHFFFAOYSA-N
Lewis structure
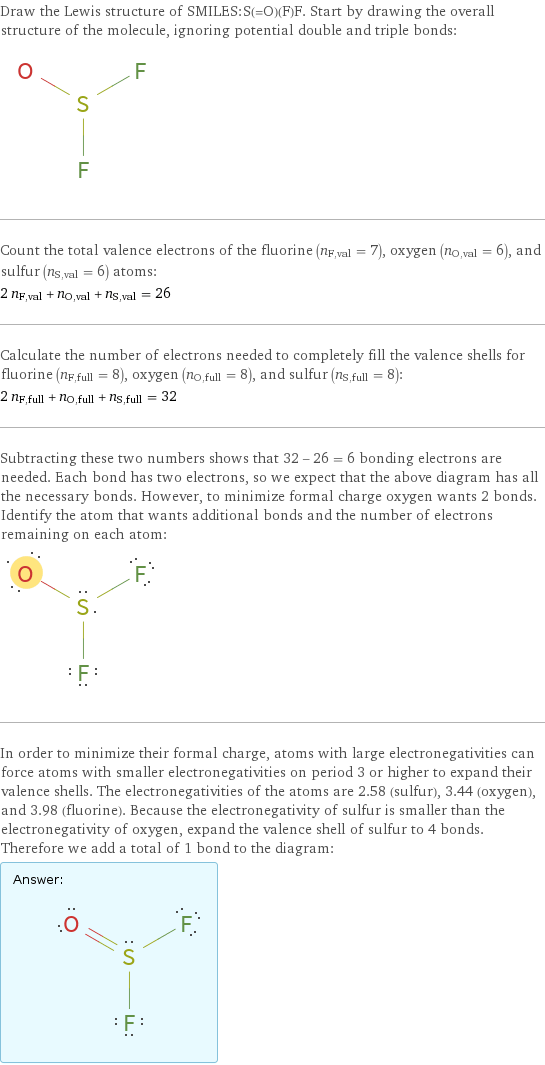
Draw the Lewis structure of SMILES:S(=O)(F)F. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the fluorine (n_F, val = 7), oxygen (n_O, val = 6), and sulfur (n_S, val = 6) atoms: 2 n_F, val + n_O, val + n_S, val = 26 Calculate the number of electrons needed to completely fill the valence shells for fluorine (n_F, full = 8), oxygen (n_O, full = 8), and sulfur (n_S, full = 8): 2 n_F, full + n_O, full + n_S, full = 32 Subtracting these two numbers shows that 32 - 26 = 6 bonding electrons are needed. Each bond has two electrons, so we expect that the above diagram has all the necessary bonds. However, to minimize formal charge oxygen wants 2 bonds. Identify the atom that wants additional bonds and the number of electrons remaining on each atom: In order to minimize their formal charge, atoms with large electronegativities can force atoms with smaller electronegativities on period 3 or higher to expand their valence shells. The electronegativities of the atoms are 2.58 (sulfur), 3.44 (oxygen), and 3.98 (fluorine). Because the electronegativity of sulfur is smaller than the electronegativity of oxygen, expand the valence shell of sulfur to 4 bonds. Therefore we add a total of 1 bond to the diagram: Answer: | |
Quantitative molecular descriptors
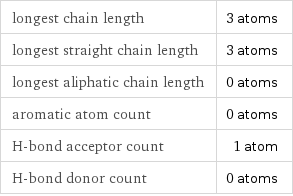
longest chain length | 3 atoms longest straight chain length | 3 atoms longest aliphatic chain length | 0 atoms aromatic atom count | 0 atoms H-bond acceptor count | 1 atom H-bond donor count | 0 atoms
Elemental composition
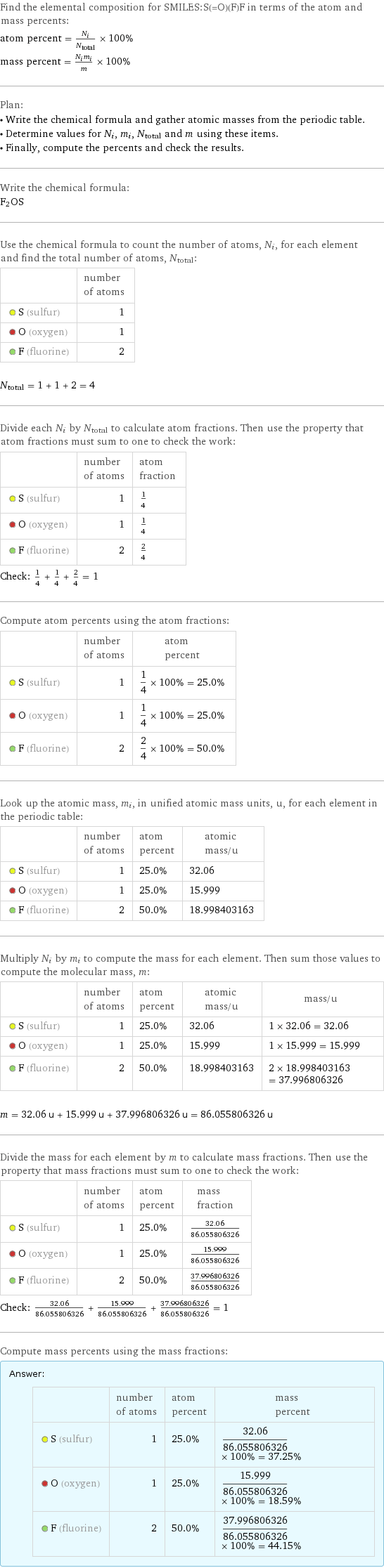
Find the elemental composition for SMILES:S(=O)(F)F in terms of the atom and mass percents: atom percent = N_i/N_total × 100% mass percent = (N_im_i)/m × 100% Plan: • Write the chemical formula and gather atomic masses from the periodic table. • Determine values for N_i, m_i, N_total and m using these items. • Finally, compute the percents and check the results. Write the chemical formula: F_2OS Use the chemical formula to count the number of atoms, N_i, for each element and find the total number of atoms, N_total: | number of atoms S (sulfur) | 1 O (oxygen) | 1 F (fluorine) | 2 N_total = 1 + 1 + 2 = 4 Divide each N_i by N_total to calculate atom fractions. Then use the property that atom fractions must sum to one to check the work: | number of atoms | atom fraction S (sulfur) | 1 | 1/4 O (oxygen) | 1 | 1/4 F (fluorine) | 2 | 2/4 Check: 1/4 + 1/4 + 2/4 = 1 Compute atom percents using the atom fractions: | number of atoms | atom percent S (sulfur) | 1 | 1/4 × 100% = 25.0% O (oxygen) | 1 | 1/4 × 100% = 25.0% F (fluorine) | 2 | 2/4 × 100% = 50.0% Look up the atomic mass, m_i, in unified atomic mass units, u, for each element in the periodic table: | number of atoms | atom percent | atomic mass/u S (sulfur) | 1 | 25.0% | 32.06 O (oxygen) | 1 | 25.0% | 15.999 F (fluorine) | 2 | 50.0% | 18.998403163 Multiply N_i by m_i to compute the mass for each element. Then sum those values to compute the molecular mass, m: | number of atoms | atom percent | atomic mass/u | mass/u S (sulfur) | 1 | 25.0% | 32.06 | 1 × 32.06 = 32.06 O (oxygen) | 1 | 25.0% | 15.999 | 1 × 15.999 = 15.999 F (fluorine) | 2 | 50.0% | 18.998403163 | 2 × 18.998403163 = 37.996806326 m = 32.06 u + 15.999 u + 37.996806326 u = 86.055806326 u Divide the mass for each element by m to calculate mass fractions. Then use the property that mass fractions must sum to one to check the work: | number of atoms | atom percent | mass fraction S (sulfur) | 1 | 25.0% | 32.06/86.055806326 O (oxygen) | 1 | 25.0% | 15.999/86.055806326 F (fluorine) | 2 | 50.0% | 37.996806326/86.055806326 Check: 32.06/86.055806326 + 15.999/86.055806326 + 37.996806326/86.055806326 = 1 Compute mass percents using the mass fractions: Answer: | | | number of atoms | atom percent | mass percent S (sulfur) | 1 | 25.0% | 32.06/86.055806326 × 100% = 37.25% O (oxygen) | 1 | 25.0% | 15.999/86.055806326 × 100% = 18.59% F (fluorine) | 2 | 50.0% | 37.996806326/86.055806326 × 100% = 44.15%
Elemental oxidation states
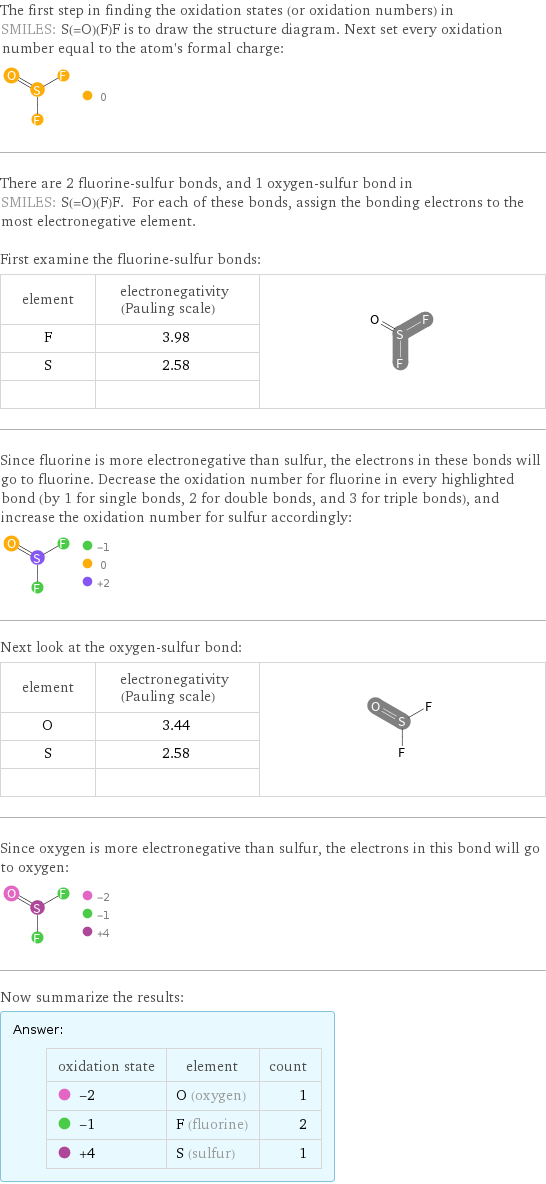
The first step in finding the oxidation states (or oxidation numbers) in SMILES: S(=O)(F)F is to draw the structure diagram. Next set every oxidation number equal to the atom's formal charge: There are 2 fluorine-sulfur bonds, and 1 oxygen-sulfur bond in SMILES: S(=O)(F)F. For each of these bonds, assign the bonding electrons to the most electronegative element. First examine the fluorine-sulfur bonds: element | electronegativity (Pauling scale) | F | 3.98 | S | 2.58 | | | Since fluorine is more electronegative than sulfur, the electrons in these bonds will go to fluorine. Decrease the oxidation number for fluorine in every highlighted bond (by 1 for single bonds, 2 for double bonds, and 3 for triple bonds), and increase the oxidation number for sulfur accordingly: Next look at the oxygen-sulfur bond: element | electronegativity (Pauling scale) | O | 3.44 | S | 2.58 | | | Since oxygen is more electronegative than sulfur, the electrons in this bond will go to oxygen: Now summarize the results: Answer: | | oxidation state | element | count -2 | O (oxygen) | 1 -1 | F (fluorine) | 2 +4 | S (sulfur) | 1
Orbital hybridization
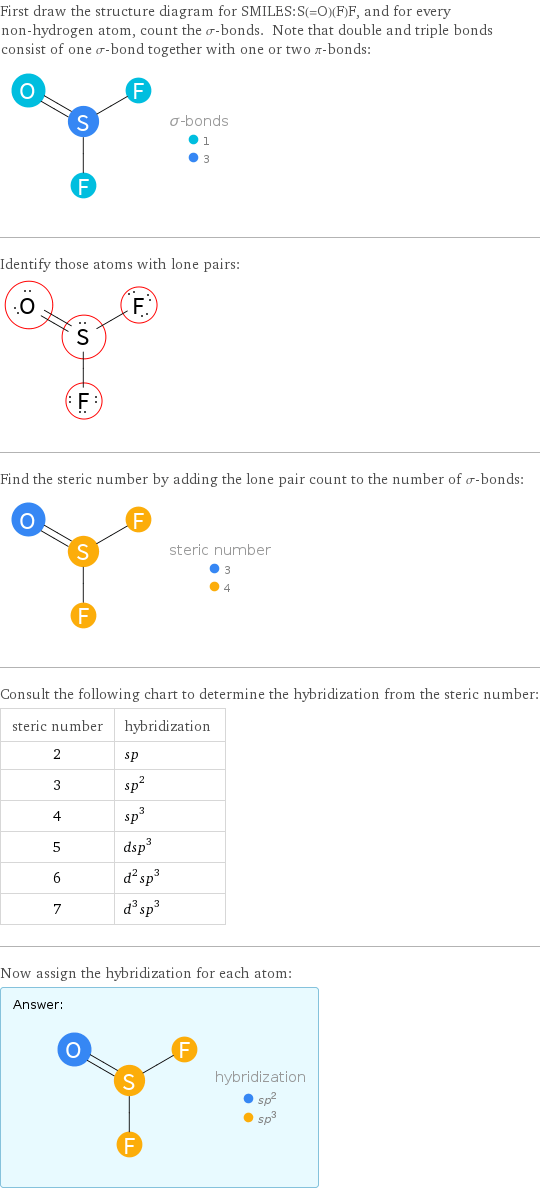
First draw the structure diagram for SMILES:S(=O)(F)F, and for every non-hydrogen atom, count the σ-bonds. Note that double and triple bonds consist of one σ-bond together with one or two π-bonds: Identify those atoms with lone pairs: Find the steric number by adding the lone pair count to the number of σ-bonds: Consult the following chart to determine the hybridization from the steric number: steric number | hybridization 2 | sp 3 | sp^2 4 | sp^3 5 | dsp^3 6 | d^2sp^3 7 | d^3sp^3 Now assign the hybridization for each atom: Answer: | |
Topological indices
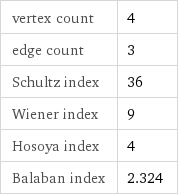
vertex count | 4 edge count | 3 Schultz index | 36 Wiener index | 9 Hosoya index | 4 Balaban index | 2.324