Input interpretation
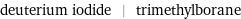
deuterium iodide | trimethylborane
Chemical names and formulas
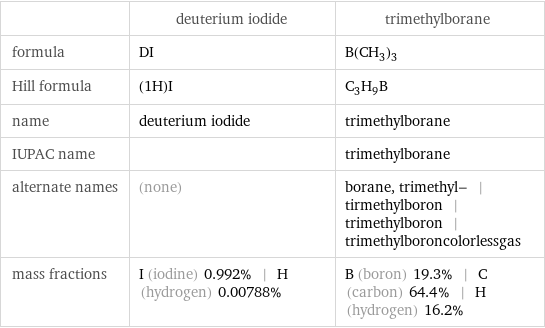
| deuterium iodide | trimethylborane formula | DI | B(CH_3)_3 Hill formula | (1H)I | C_3H_9B name | deuterium iodide | trimethylborane IUPAC name | | trimethylborane alternate names | (none) | borane, trimethyl- | tirmethylboron | trimethylboron | trimethylboroncolorlessgas mass fractions | I (iodine) 0.992% | H (hydrogen) 0.00788% | B (boron) 19.3% | C (carbon) 64.4% | H (hydrogen) 16.2%
Structure diagrams
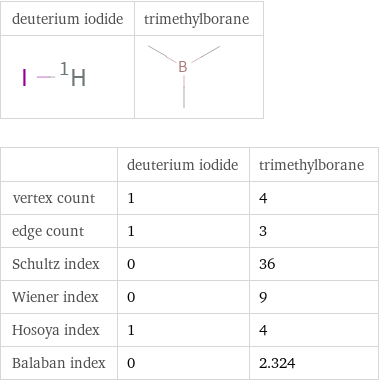
| deuterium iodide | trimethylborane vertex count | 1 | 4 edge count | 1 | 3 Schultz index | 0 | 36 Wiener index | 0 | 9 Hosoya index | 1 | 4 Balaban index | 0 | 2.324
3D structure
3D structure
Basic properties
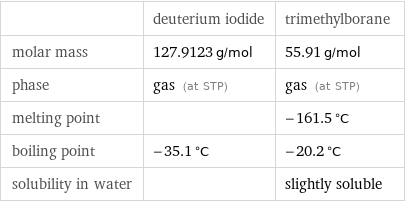
| deuterium iodide | trimethylborane molar mass | 127.9123 g/mol | 55.91 g/mol phase | gas (at STP) | gas (at STP) melting point | | -161.5 °C boiling point | -35.1 °C | -20.2 °C solubility in water | | slightly soluble
Units

Gas properties (at STP)

| trimethylborane vapor density | 907.
Thermodynamic properties

| trimethylborane specific heat of vaporization | 0.458 kJ/g (kilojoules per gram)
Non-standard atom properties
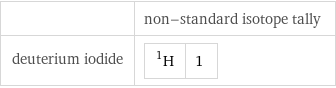
| non-standard isotope tally deuterium iodide | H-1 | 1