Input interpretation
aluminum antimonide
Chemical names and formulas
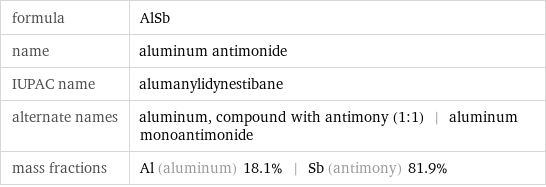
formula | AlSb name | aluminum antimonide IUPAC name | alumanylidynestibane alternate names | aluminum, compound with antimony (1:1) | aluminum monoantimonide mass fractions | Al (aluminum) 18.1% | Sb (antimony) 81.9%
Lewis structure

Draw the Lewis structure of aluminum antimonide. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the aluminum (n_Al, val = 3) and antimony (n_Sb, val = 5) atoms: n_Al, val + n_Sb, val = 8 Calculate the number of electrons needed to completely fill the valence shells for aluminum (n_Al, full = 6) and antimony (n_Sb, full = 8): n_Al, full + n_Sb, full = 14 Subtracting these two numbers shows that 14 - 8 = 6 bonding electrons are needed. Each bond has two electrons, so in addition to the 1 bond already present in the diagram add 2 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: Fill in the 2 bonds by pairing electrons between adjacent highlighted atoms: Answer: | |
Basic properties
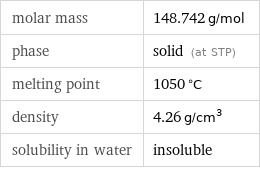
molar mass | 148.742 g/mol phase | solid (at STP) melting point | 1050 °C density | 4.26 g/cm^3 solubility in water | insoluble
Units

Solid properties (at STP)

density | 4.26 g/cm^3
Units

Chemical identifiers
![CAS number | 25152-52-7 PubChem CID number | 91307 SMILES identifier | [Al]#[Sb] InChI identifier | InChI=1/Al.Sb/rAlSb/c1-2 EU number | 246-667-3](../image_source/b019f8bf02c7657cececd6bcca2cac41.png)
CAS number | 25152-52-7 PubChem CID number | 91307 SMILES identifier | [Al]#[Sb] InChI identifier | InChI=1/Al.Sb/rAlSb/c1-2 EU number | 246-667-3