Input interpretation

naturally occurring elements | phase at STP
Summary
gas | liquid | solid
Categorized list

gas | argon, chlorine, fluorine, helium, hydrogen, krypton, neon, nitrogen, oxygen, radon, xenon liquid | bromine, mercury solid | actinium, aluminum, antimony, arsenic, barium, beryllium, bismuth, boron, cadmium, calcium, carbon, cerium, cesium, chromium, cobalt, copper, dysprosium, erbium, europium, francium, ...
Thermodynamic properties
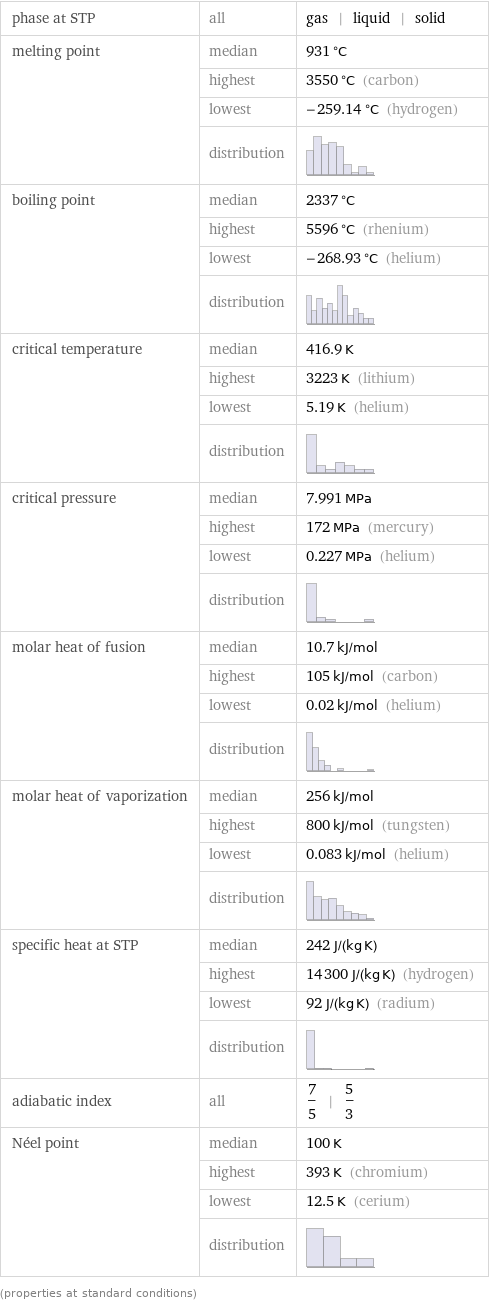
phase at STP | all | gas | liquid | solid melting point | median | 931 °C | highest | 3550 °C (carbon) | lowest | -259.14 °C (hydrogen) | distribution | boiling point | median | 2337 °C | highest | 5596 °C (rhenium) | lowest | -268.93 °C (helium) | distribution | critical temperature | median | 416.9 K | highest | 3223 K (lithium) | lowest | 5.19 K (helium) | distribution | critical pressure | median | 7.991 MPa | highest | 172 MPa (mercury) | lowest | 0.227 MPa (helium) | distribution | molar heat of fusion | median | 10.7 kJ/mol | highest | 105 kJ/mol (carbon) | lowest | 0.02 kJ/mol (helium) | distribution | molar heat of vaporization | median | 256 kJ/mol | highest | 800 kJ/mol (tungsten) | lowest | 0.083 kJ/mol (helium) | distribution | specific heat at STP | median | 242 J/(kg K) | highest | 14300 J/(kg K) (hydrogen) | lowest | 92 J/(kg K) (radium) | distribution | adiabatic index | all | 7/5 | 5/3 Néel point | median | 100 K | highest | 393 K (chromium) | lowest | 12.5 K (cerium) | distribution | (properties at standard conditions)