Input interpretation
vanadium(III) oxide
Chemical names and formulas

formula | V_2O_3 Hill formula | O_3V_2 name | vanadium(III) oxide mass fractions | O (oxygen) 32% | V (vanadium) 68%
Structure diagram

Structure diagram
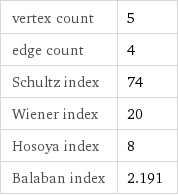
vertex count | 5 edge count | 4 Schultz index | 74 Wiener index | 20 Hosoya index | 8 Balaban index | 2.191
Basic properties
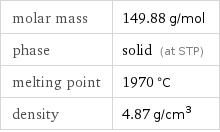
molar mass | 149.88 g/mol phase | solid (at STP) melting point | 1970 °C density | 4.87 g/cm^3
Units

Solid properties (at STP)

density | 4.87 g/cm^3
Units

Thermodynamic properties
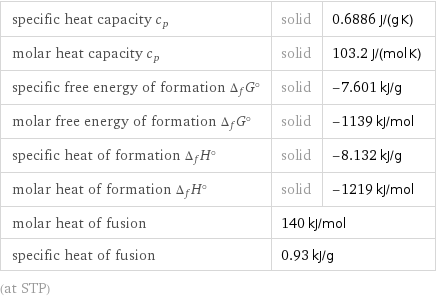
specific heat capacity c_p | solid | 0.6886 J/(g K) molar heat capacity c_p | solid | 103.2 J/(mol K) specific free energy of formation Δ_fG° | solid | -7.601 kJ/g molar free energy of formation Δ_fG° | solid | -1139 kJ/mol specific heat of formation Δ_fH° | solid | -8.132 kJ/g molar heat of formation Δ_fH° | solid | -1219 kJ/mol molar heat of fusion | 140 kJ/mol | specific heat of fusion | 0.93 kJ/g | (at STP)
Chemical identifiers
![CAS number | 1314-34-7 PubChem CID number | 518710 PubChem SID number | 24852926 SMILES identifier | O=[V]O[V]=O InChI identifier | InChI=1/3O.2V/rO3V2/c1-4-3-5-2 RTECS number | YW3050000 MDL number | MFCD00011455](../image_source/7d7a3e6c29d9799f895d79b28dc9b17e.png)
CAS number | 1314-34-7 PubChem CID number | 518710 PubChem SID number | 24852926 SMILES identifier | O=[V]O[V]=O InChI identifier | InChI=1/3O.2V/rO3V2/c1-4-3-5-2 RTECS number | YW3050000 MDL number | MFCD00011455