Input interpretation
cadmium hydroxide
Chemical names and formulas

formula | Cd(OH)_2 Hill formula | CdH_2O_2 name | cadmium hydroxide alternate names | cadmium dihydrate mass fractions | Cd (cadmium) 76.8% | H (hydrogen) 1.38% | O (oxygen) 21.9%
Structure diagram
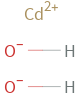
Structure diagram
Basic properties
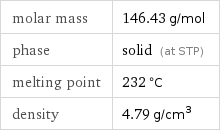
molar mass | 146.43 g/mol phase | solid (at STP) melting point | 232 °C density | 4.79 g/cm^3
Units

Solid properties (at STP)

density | 4.79 g/cm^3
Units

Thermodynamic properties
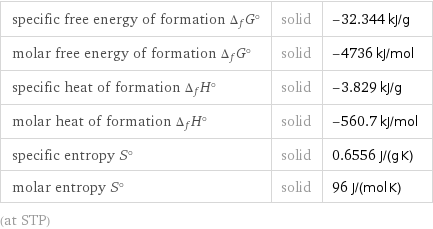
specific free energy of formation Δ_fG° | solid | -32.344 kJ/g molar free energy of formation Δ_fG° | solid | -4736 kJ/mol specific heat of formation Δ_fH° | solid | -3.829 kJ/g molar heat of formation Δ_fH° | solid | -560.7 kJ/mol specific entropy S° | solid | 0.6556 J/(g K) molar entropy S° | solid | 96 J/(mol K) (at STP)
Chemical identifiers
![CAS number | 21041-95-2 SMILES identifier | [Cd+2].[OH-].[OH-] RTECS number | EV1260000 MDL number | MFCD00015995](../image_source/5efa607b6002cb26a2a73d73ee4a0cd9.png)
CAS number | 21041-95-2 SMILES identifier | [Cd+2].[OH-].[OH-] RTECS number | EV1260000 MDL number | MFCD00015995