Input interpretation
barium (chemical element) | bismuth (chemical element) | dysprosium (chemical element)
Periodic table location
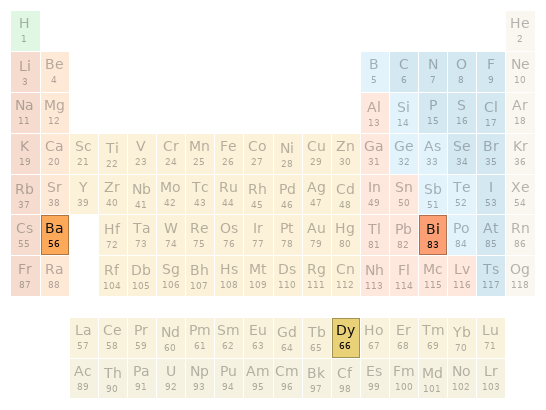
Periodic table location
Images

Images
Basic elemental properties
![| barium | bismuth | dysprosium atomic symbol | Ba | Bi | Dy atomic number | 56 | 83 | 66 short electronic configuration | [Xe]6s^2 | [Xe]6s^24f^145d^106p^3 | [Xe]6s^24f^10 Aufbau diagram | 6s | 6p 5d 4f 6s | 4f 6s block | s | p | f group | 2 | 15 | period | 6 | 6 | 6 atomic mass | 137.327 u | 208.9804 u | 162.5 u half-life | (stable) | 1.9×10^19 yr | (stable)](../image_source/f5a30ba668d88a346904b88b03b05061.png)
| barium | bismuth | dysprosium atomic symbol | Ba | Bi | Dy atomic number | 56 | 83 | 66 short electronic configuration | [Xe]6s^2 | [Xe]6s^24f^145d^106p^3 | [Xe]6s^24f^10 Aufbau diagram | 6s | 6p 5d 4f 6s | 4f 6s block | s | p | f group | 2 | 15 | period | 6 | 6 | 6 atomic mass | 137.327 u | 208.9804 u | 162.5 u half-life | (stable) | 1.9×10^19 yr | (stable)
Thermodynamic properties
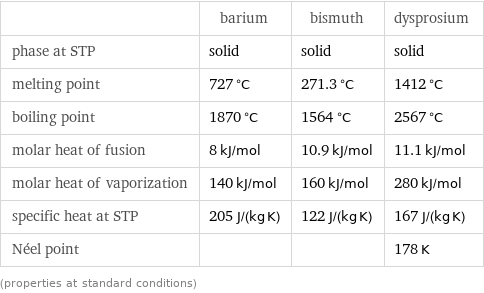
| barium | bismuth | dysprosium phase at STP | solid | solid | solid melting point | 727 °C | 271.3 °C | 1412 °C boiling point | 1870 °C | 1564 °C | 2567 °C molar heat of fusion | 8 kJ/mol | 10.9 kJ/mol | 11.1 kJ/mol molar heat of vaporization | 140 kJ/mol | 160 kJ/mol | 280 kJ/mol specific heat at STP | 205 J/(kg K) | 122 J/(kg K) | 167 J/(kg K) Néel point | | | 178 K (properties at standard conditions)
Material properties
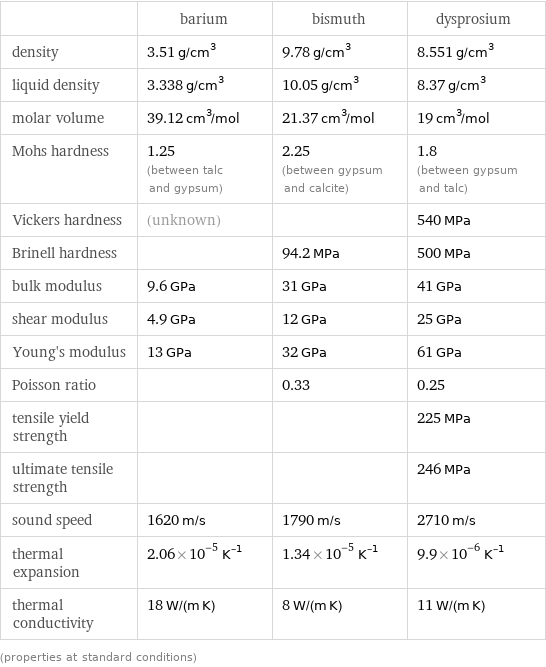
| barium | bismuth | dysprosium density | 3.51 g/cm^3 | 9.78 g/cm^3 | 8.551 g/cm^3 liquid density | 3.338 g/cm^3 | 10.05 g/cm^3 | 8.37 g/cm^3 molar volume | 39.12 cm^3/mol | 21.37 cm^3/mol | 19 cm^3/mol Mohs hardness | 1.25 (between talc and gypsum) | 2.25 (between gypsum and calcite) | 1.8 (between gypsum and talc) Vickers hardness | (unknown) | | 540 MPa Brinell hardness | | 94.2 MPa | 500 MPa bulk modulus | 9.6 GPa | 31 GPa | 41 GPa shear modulus | 4.9 GPa | 12 GPa | 25 GPa Young's modulus | 13 GPa | 32 GPa | 61 GPa Poisson ratio | | 0.33 | 0.25 tensile yield strength | | | 225 MPa ultimate tensile strength | | | 246 MPa sound speed | 1620 m/s | 1790 m/s | 2710 m/s thermal expansion | 2.06×10^-5 K^(-1) | 1.34×10^-5 K^(-1) | 9.9×10^-6 K^(-1) thermal conductivity | 18 W/(m K) | 8 W/(m K) | 11 W/(m K) (properties at standard conditions)
Electromagnetic properties
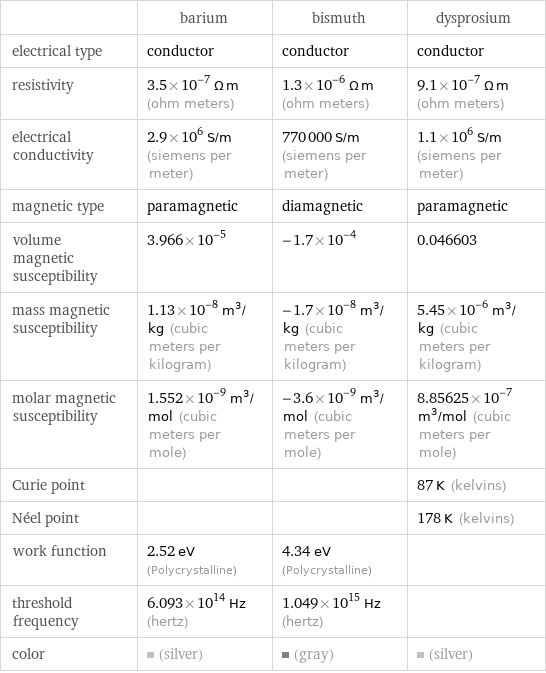
| barium | bismuth | dysprosium electrical type | conductor | conductor | conductor resistivity | 3.5×10^-7 Ω m (ohm meters) | 1.3×10^-6 Ω m (ohm meters) | 9.1×10^-7 Ω m (ohm meters) electrical conductivity | 2.9×10^6 S/m (siemens per meter) | 770000 S/m (siemens per meter) | 1.1×10^6 S/m (siemens per meter) magnetic type | paramagnetic | diamagnetic | paramagnetic volume magnetic susceptibility | 3.966×10^-5 | -1.7×10^-4 | 0.046603 mass magnetic susceptibility | 1.13×10^-8 m^3/kg (cubic meters per kilogram) | -1.7×10^-8 m^3/kg (cubic meters per kilogram) | 5.45×10^-6 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | 1.552×10^-9 m^3/mol (cubic meters per mole) | -3.6×10^-9 m^3/mol (cubic meters per mole) | 8.85625×10^-7 m^3/mol (cubic meters per mole) Curie point | | | 87 K (kelvins) Néel point | | | 178 K (kelvins) work function | 2.52 eV (Polycrystalline) | 4.34 eV (Polycrystalline) | threshold frequency | 6.093×10^14 Hz (hertz) | 1.049×10^15 Hz (hertz) | color | (silver) | (gray) | (silver)
Reactivity
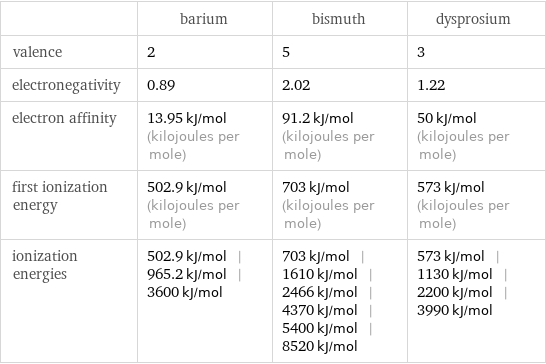
| barium | bismuth | dysprosium valence | 2 | 5 | 3 electronegativity | 0.89 | 2.02 | 1.22 electron affinity | 13.95 kJ/mol (kilojoules per mole) | 91.2 kJ/mol (kilojoules per mole) | 50 kJ/mol (kilojoules per mole) first ionization energy | 502.9 kJ/mol (kilojoules per mole) | 703 kJ/mol (kilojoules per mole) | 573 kJ/mol (kilojoules per mole) ionization energies | 502.9 kJ/mol | 965.2 kJ/mol | 3600 kJ/mol | 703 kJ/mol | 1610 kJ/mol | 2466 kJ/mol | 4370 kJ/mol | 5400 kJ/mol | 8520 kJ/mol | 573 kJ/mol | 1130 kJ/mol | 2200 kJ/mol | 3990 kJ/mol
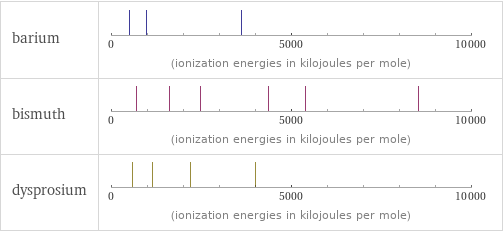
Reactivity
Atomic properties
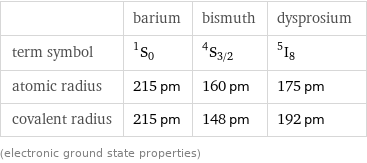
| barium | bismuth | dysprosium term symbol | ^1S_0 | ^4S_(3/2) | ^5I_8 atomic radius | 215 pm | 160 pm | 175 pm covalent radius | 215 pm | 148 pm | 192 pm (electronic ground state properties)
Abundances
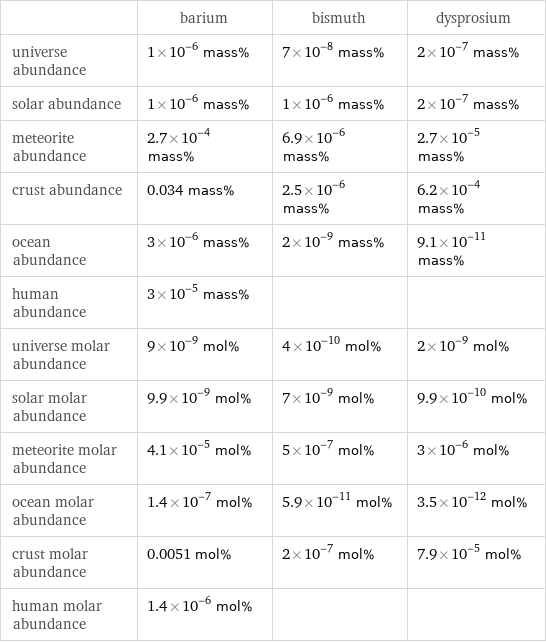
| barium | bismuth | dysprosium universe abundance | 1×10^-6 mass% | 7×10^-8 mass% | 2×10^-7 mass% solar abundance | 1×10^-6 mass% | 1×10^-6 mass% | 2×10^-7 mass% meteorite abundance | 2.7×10^-4 mass% | 6.9×10^-6 mass% | 2.7×10^-5 mass% crust abundance | 0.034 mass% | 2.5×10^-6 mass% | 6.2×10^-4 mass% ocean abundance | 3×10^-6 mass% | 2×10^-9 mass% | 9.1×10^-11 mass% human abundance | 3×10^-5 mass% | | universe molar abundance | 9×10^-9 mol% | 4×10^-10 mol% | 2×10^-9 mol% solar molar abundance | 9.9×10^-9 mol% | 7×10^-9 mol% | 9.9×10^-10 mol% meteorite molar abundance | 4.1×10^-5 mol% | 5×10^-7 mol% | 3×10^-6 mol% ocean molar abundance | 1.4×10^-7 mol% | 5.9×10^-11 mol% | 3.5×10^-12 mol% crust molar abundance | 0.0051 mol% | 2×10^-7 mol% | 7.9×10^-5 mol% human molar abundance | 1.4×10^-6 mol% | |
Nuclear properties
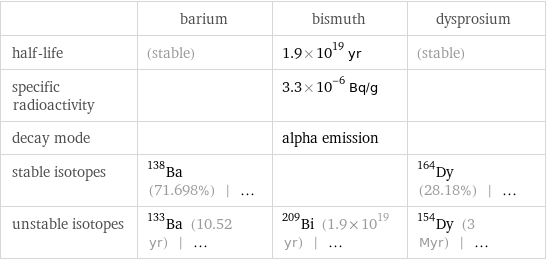
| barium | bismuth | dysprosium half-life | (stable) | 1.9×10^19 yr | (stable) specific radioactivity | | 3.3×10^-6 Bq/g | decay mode | | alpha emission | stable isotopes | Ba-138 (71.698%) | ... | | Dy-164 (28.18%) | ... unstable isotopes | Ba-133 (10.52 yr) | ... | Bi-209 (1.9×10^19 yr) | ... | Dy-154 (3 Myr) | ...
Units