Input interpretation
ammonium nitrate
Chemical names and formulas

formula | NH_4NO_3 Hill formula | H_4N_2O_3 name | ammonium nitrate alternate names | ammonia; nitric acid | ammonium nitricum | azane; nitric acid | nitric acid, ammonium salt mass fractions | H (hydrogen) 5.04% | N (nitrogen) 35% | O (oxygen) 60%
Structure diagram
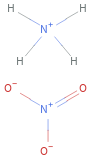
Structure diagram
Basic properties
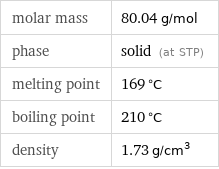
molar mass | 80.04 g/mol phase | solid (at STP) melting point | 169 °C boiling point | 210 °C density | 1.73 g/cm^3
Units

Solid properties (at STP)
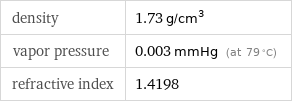
density | 1.73 g/cm^3 vapor pressure | 0.003 mmHg (at 79 °C) refractive index | 1.4198
Units

Thermodynamic properties
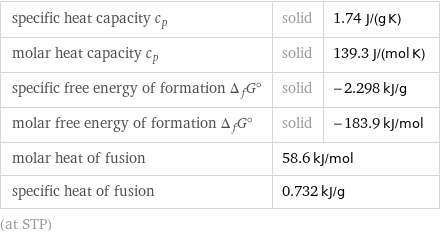
specific heat capacity c_p | solid | 1.74 J/(g K) molar heat capacity c_p | solid | 139.3 J/(mol K) specific free energy of formation Δ_fG° | solid | -2.298 kJ/g molar free energy of formation Δ_fG° | solid | -183.9 kJ/mol molar heat of fusion | 58.6 kJ/mol | specific heat of fusion | 0.732 kJ/g | (at STP)
Chemical identifiers
([O-])[O-].[NH4+] RTECS number | BR9050000 MDL number | MFCD00011425](../image_source/2bdd28017457f8a2375a0922fd26c2fd.png)
CAS number | 6484-52-2 SMILES identifier | [N+](=O)([O-])[O-].[NH4+] RTECS number | BR9050000 MDL number | MFCD00011425
NFPA label

NFPA label
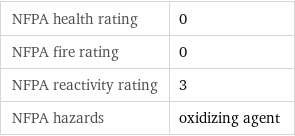
NFPA health rating | 0 NFPA fire rating | 0 NFPA reactivity rating | 3 NFPA hazards | oxidizing agent
Toxicity properties

odor | odorless

RTECS classes | other