Input interpretation
neodymium(III) sulfate octahydrate | americium(IV) oxide
Chemical names and formulas
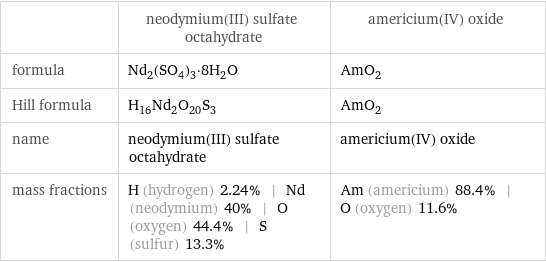
| neodymium(III) sulfate octahydrate | americium(IV) oxide formula | Nd_2(SO_4)_3·8H_2O | AmO_2 Hill formula | H_16Nd_2O_20S_3 | AmO_2 name | neodymium(III) sulfate octahydrate | americium(IV) oxide mass fractions | H (hydrogen) 2.24% | Nd (neodymium) 40% | O (oxygen) 44.4% | S (sulfur) 13.3% | Am (americium) 88.4% | O (oxygen) 11.6%
Structure diagrams
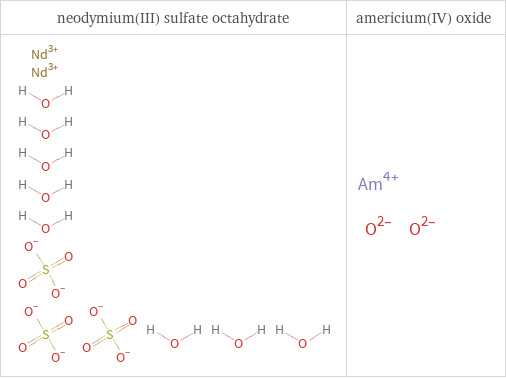
Structure diagrams
Basic properties
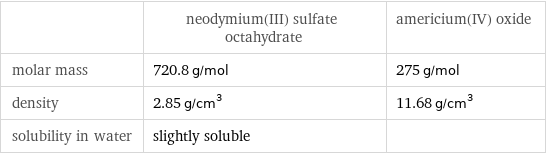
| neodymium(III) sulfate octahydrate | americium(IV) oxide molar mass | 720.8 g/mol | 275 g/mol density | 2.85 g/cm^3 | 11.68 g/cm^3 solubility in water | slightly soluble |
Units
