Input interpretation
inorganic salts
Members

ammonium bisulfate | ammonium nitrate | beryllium chloride | cesium fluoride | lithium bromide | lithium iodide | lithium sulfate | nickel(II) nitrate | potassium chloride | potassium iodide | rubidium bromide | rubidium chloride | rubidium fluoride | rubidium nitrate | sodium bromate | sodium bromide | sodium chloride | sodium sulfate | sodium sulfide | sodium hyposulfite (total: 20)
Basic properties
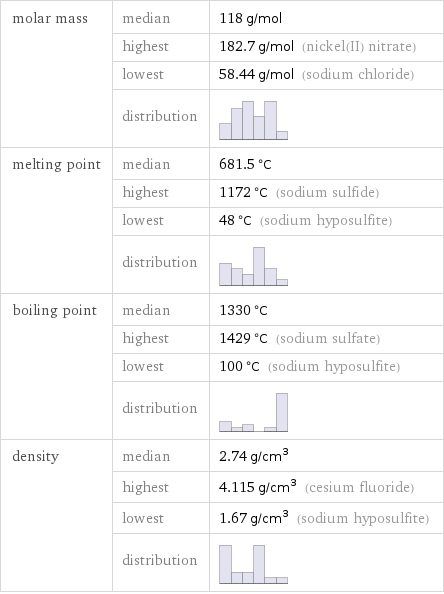
molar mass | median | 118 g/mol | highest | 182.7 g/mol (nickel(II) nitrate) | lowest | 58.44 g/mol (sodium chloride) | distribution | melting point | median | 681.5 °C | highest | 1172 °C (sodium sulfide) | lowest | 48 °C (sodium hyposulfite) | distribution | boiling point | median | 1330 °C | highest | 1429 °C (sodium sulfate) | lowest | 100 °C (sodium hyposulfite) | distribution | density | median | 2.74 g/cm^3 | highest | 4.115 g/cm^3 (cesium fluoride) | lowest | 1.67 g/cm^3 (sodium hyposulfite) | distribution |
Units
