Input interpretation

sodium fluoride | element types
Result
fluorine | sodium
Periodic table location
Periodic table location
Images

Images
Basic elemental properties
![| fluorine | sodium atomic symbol | F | Na atomic number | 9 | 11 short electronic configuration | [He]2s^22p^5 | [Ne]3s^1 Aufbau diagram | 2p 2s | 3s block | p | s group | 17 | 1 period | 2 | 3 atomic mass | 18.998403163 u | 22.98976928 u half-life | (stable) | (stable)](../image_source/39fab7281a168bd0cc98800a16836f4f.png)
| fluorine | sodium atomic symbol | F | Na atomic number | 9 | 11 short electronic configuration | [He]2s^22p^5 | [Ne]3s^1 Aufbau diagram | 2p 2s | 3s block | p | s group | 17 | 1 period | 2 | 3 atomic mass | 18.998403163 u | 22.98976928 u half-life | (stable) | (stable)
Thermodynamic properties
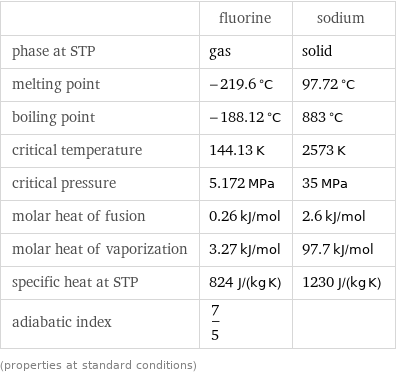
| fluorine | sodium phase at STP | gas | solid melting point | -219.6 °C | 97.72 °C boiling point | -188.12 °C | 883 °C critical temperature | 144.13 K | 2573 K critical pressure | 5.172 MPa | 35 MPa molar heat of fusion | 0.26 kJ/mol | 2.6 kJ/mol molar heat of vaporization | 3.27 kJ/mol | 97.7 kJ/mol specific heat at STP | 824 J/(kg K) | 1230 J/(kg K) adiabatic index | 7/5 | (properties at standard conditions)
Material properties
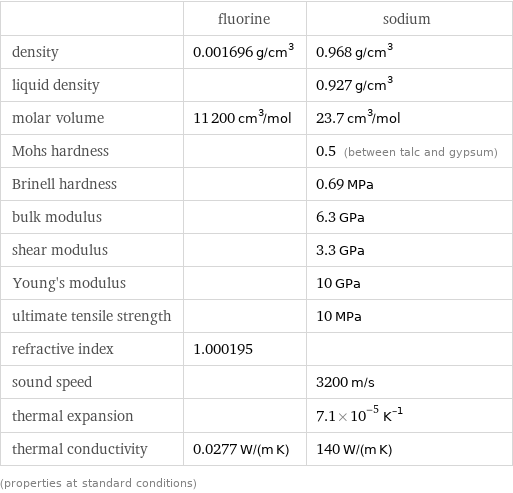
| fluorine | sodium density | 0.001696 g/cm^3 | 0.968 g/cm^3 liquid density | | 0.927 g/cm^3 molar volume | 11200 cm^3/mol | 23.7 cm^3/mol Mohs hardness | | 0.5 (between talc and gypsum) Brinell hardness | | 0.69 MPa bulk modulus | | 6.3 GPa shear modulus | | 3.3 GPa Young's modulus | | 10 GPa ultimate tensile strength | | 10 MPa refractive index | 1.000195 | sound speed | | 3200 m/s thermal expansion | | 7.1×10^-5 K^(-1) thermal conductivity | 0.0277 W/(m K) | 140 W/(m K) (properties at standard conditions)
Electromagnetic properties
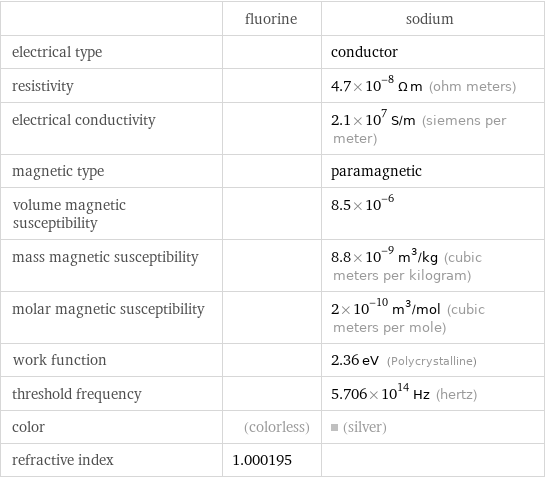
| fluorine | sodium electrical type | | conductor resistivity | | 4.7×10^-8 Ω m (ohm meters) electrical conductivity | | 2.1×10^7 S/m (siemens per meter) magnetic type | | paramagnetic volume magnetic susceptibility | | 8.5×10^-6 mass magnetic susceptibility | | 8.8×10^-9 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | | 2×10^-10 m^3/mol (cubic meters per mole) work function | | 2.36 eV (Polycrystalline) threshold frequency | | 5.706×10^14 Hz (hertz) color | (colorless) | (silver) refractive index | 1.000195 |
Reactivity
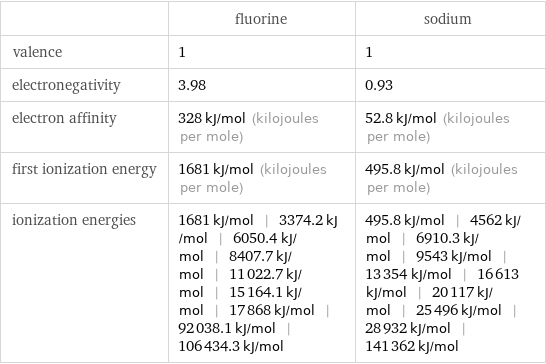
| fluorine | sodium valence | 1 | 1 electronegativity | 3.98 | 0.93 electron affinity | 328 kJ/mol (kilojoules per mole) | 52.8 kJ/mol (kilojoules per mole) first ionization energy | 1681 kJ/mol (kilojoules per mole) | 495.8 kJ/mol (kilojoules per mole) ionization energies | 1681 kJ/mol | 3374.2 kJ/mol | 6050.4 kJ/mol | 8407.7 kJ/mol | 11022.7 kJ/mol | 15164.1 kJ/mol | 17868 kJ/mol | 92038.1 kJ/mol | 106434.3 kJ/mol | 495.8 kJ/mol | 4562 kJ/mol | 6910.3 kJ/mol | 9543 kJ/mol | 13354 kJ/mol | 16613 kJ/mol | 20117 kJ/mol | 25496 kJ/mol | 28932 kJ/mol | 141362 kJ/mol
Reactivity
Atomic properties
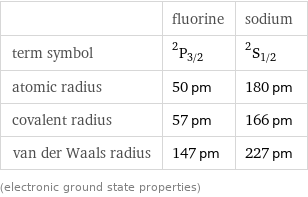
| fluorine | sodium term symbol | ^2P_(3/2) | ^2S_(1/2) atomic radius | 50 pm | 180 pm covalent radius | 57 pm | 166 pm van der Waals radius | 147 pm | 227 pm (electronic ground state properties)
Abundances
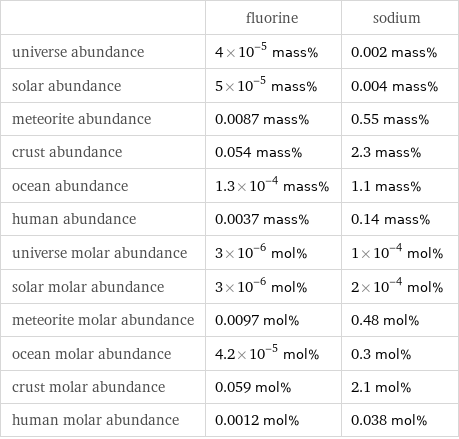
| fluorine | sodium universe abundance | 4×10^-5 mass% | 0.002 mass% solar abundance | 5×10^-5 mass% | 0.004 mass% meteorite abundance | 0.0087 mass% | 0.55 mass% crust abundance | 0.054 mass% | 2.3 mass% ocean abundance | 1.3×10^-4 mass% | 1.1 mass% human abundance | 0.0037 mass% | 0.14 mass% universe molar abundance | 3×10^-6 mol% | 1×10^-4 mol% solar molar abundance | 3×10^-6 mol% | 2×10^-4 mol% meteorite molar abundance | 0.0097 mol% | 0.48 mol% ocean molar abundance | 4.2×10^-5 mol% | 0.3 mol% crust molar abundance | 0.059 mol% | 2.1 mol% human molar abundance | 0.0012 mol% | 0.038 mol%
Nuclear properties

| fluorine | sodium half-life | (stable) | (stable) stable isotopes | F-19 (100%) | Na-23 (100%) nuclear spin | F-14: 2^- | F-15: 1/2^+ | F-16: 0^- | F-17: 5/2^+ | F-18: 1^+ | F-19: 1/2^+ | F-20: 2^+ | F-21: 5/2^+ | F-22: 4^+ | F-23: 5/2^+ | F-25: 5/2^+ | F-26: 1^+ | F-27: 5/2^+ | F-29: 5/2^+ | F-31: 5/2^+ | Na-18: 1^- | Na-19: 5/2^+ | Na-20: 2^+ | Na-21: 3/2^+ | Na-22: 3^+ | Na-23: 3/2^+ | Na-24: 4^+ | Na-25: 5/2^+ | Na-26: 3^+ | Na-27: 5/2^+ | Na-28: 1^+ | Na-29: 3/2^+ | Na-30: 2^+ | Na-31: 3/2^+ | Na-33: 3/2^+ | Na-34: 1^+ | Na-35: 3/2^+ | Na-37: 3/2^+ unstable isotopes | F-18 (109.771 min) | F-17 (64.49 s) | F-20 (11.07 s) | F-22 (4.23 s) | F-21 (4.158 s) | F-23 (2.23 s) | F-24 (390 ms) | F-25 (50 ms) | F-26 (9.6 ms) | F-27 (5 ms) | F-29 (2.5 ms) | F-30 (260 ns) | F-31 (250 ns) | F-28 (40 ns) | F-16 (11 zs) | F-15 (0.46 zs) | Na-22 (2.6027 yr) | Na-24 (14.997 h) | Na-25 (59.1 s) | Na-21 (22.49 s) | Na-26 (1.077 s) | Na-20 (447.9 ms) | Na-27 (301 ms) | Na-30 (48.4 ms) | Na-29 (44.9 ms) | Na-28 (30.5 ms) | Na-31 (17 ms) | Na-32 (13.2 ms) | Na-33 (8 ms) | Na-34 (5.5 ms) | Na-35 (1.5 ms) | Na-36 (180 ns) | Na-37 (60 ns) | Na-19 (40 ns) | Na-18 (1.3 zs) neutron cross-section | 0.0096 b | 0.53 b neutron mass absorption | 2×10^-5 m^2/kg | 7×10^-4 m^2/kg
Identifiers
| fluorine | sodium CAS number | 7782-41-4 | 7440-23-5 PubChem CID number | 24524 | 5360545 Gmelin number | 16281 |