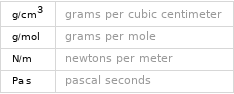Input interpretation
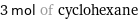
3 mol of cyclohexane
Basic properties for 3 mol
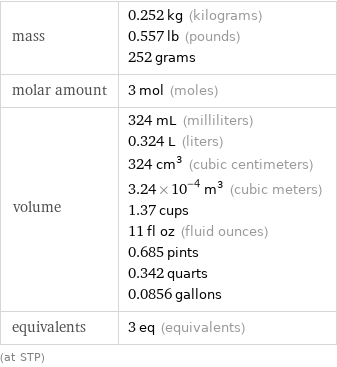
mass | 0.252 kg (kilograms) 0.557 lb (pounds) 252 grams molar amount | 3 mol (moles) volume | 324 mL (milliliters) 0.324 L (liters) 324 cm^3 (cubic centimeters) 3.24×10^-4 m^3 (cubic meters) 1.37 cups 11 fl oz (fluid ounces) 0.685 pints 0.342 quarts 0.0856 gallons equivalents | 3 eq (equivalents) (at STP)
Thermodynamic properties for 3 mol

enthalpy of hydration | -90 kJ (kilojoules) | heat capacity C_p | liquid | 464.7 J/K heat of formation Δ_fH° | gaseous | -370.2 kJ latent heat of vaporization | 99.3 kJ (kilojoules) | latent heat of fusion | 8.04 kJ (kilojoules) |
Units
Energy vs. temperature for 3 mol
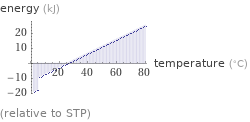
(relative to STP)
Units

Phase change energies for 3 mol from 25 °C

energy required to heat to boiling point | 25.9 kJ (kilojoules) energy required to convert to vapor | 99.3 kJ (kilojoules) energy required to heat to boiling point and convert to vapor | 125 kJ (kilojoules) energy released from cooling to freezing point | 9.06 kJ (kilojoules) energy released from converting to solid | 8.04 kJ (kilojoules) energy released from cooling to freezing point and converting to solid | 17.1 kJ (kilojoules)
Corresponding quantities
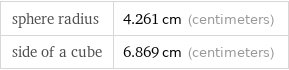
sphere radius | 4.261 cm (centimeters) side of a cube | 6.869 cm (centimeters)
Mass composition for 3 mol
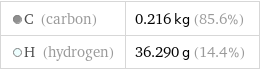
C (carbon) | 0.216 kg (85.6%) H (hydrogen) | 36.290 g (14.4%)
Mass composition for 3 mol
Lewis structure
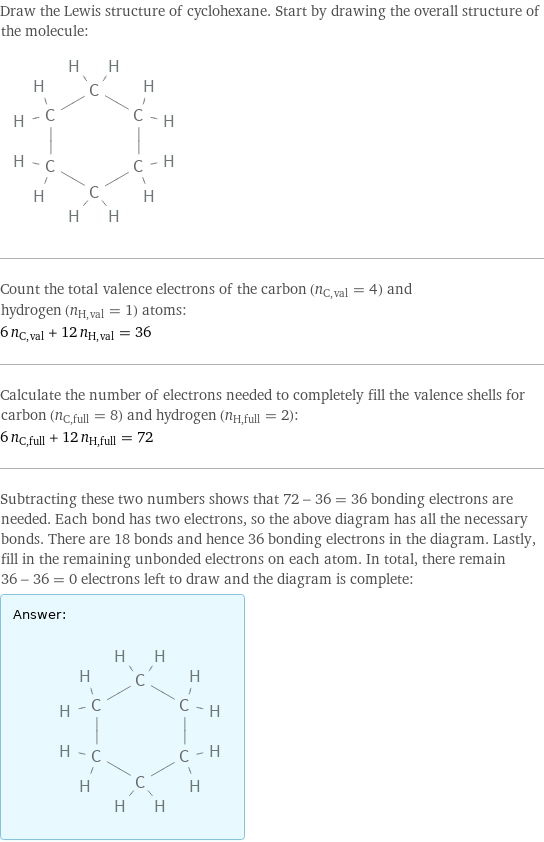
Draw the Lewis structure of cyclohexane. Start by drawing the overall structure of the molecule: Count the total valence electrons of the carbon (n_C, val = 4) and hydrogen (n_H, val = 1) atoms: 6 n_C, val + 12 n_H, val = 36 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8) and hydrogen (n_H, full = 2): 6 n_C, full + 12 n_H, full = 72 Subtracting these two numbers shows that 72 - 36 = 36 bonding electrons are needed. Each bond has two electrons, so the above diagram has all the necessary bonds. There are 18 bonds and hence 36 bonding electrons in the diagram. Lastly, fill in the remaining unbonded electrons on each atom. In total, there remain 36 - 36 = 0 electrons left to draw and the diagram is complete: Answer: | |
Chemical names and formulas
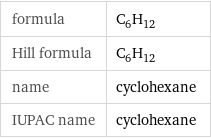
formula | C_6H_12 Hill formula | C_6H_12 name | cyclohexane IUPAC name | cyclohexane
Substance properties
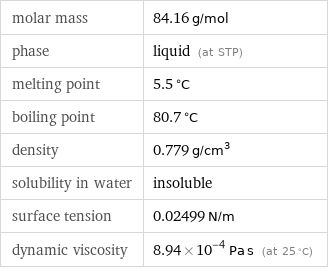
molar mass | 84.16 g/mol phase | liquid (at STP) melting point | 5.5 °C boiling point | 80.7 °C density | 0.779 g/cm^3 solubility in water | insoluble surface tension | 0.02499 N/m dynamic viscosity | 8.94×10^-4 Pa s (at 25 °C)
Units