Input interpretation
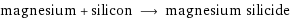
magnesium + silicon ⟶ magnesium silicide
Balanced equation

Balance the chemical equation algebraically: + ⟶ Add stoichiometric coefficients, c_i, to the reactants and products: c_1 + c_2 ⟶ c_3 Set the number of atoms in the reactants equal to the number of atoms in the products for Mg and Si: Mg: | c_1 = 2 c_3 Si: | c_2 = c_3 Since the coefficients are relative quantities and underdetermined, choose a coefficient to set arbitrarily. To keep the coefficients small, the arbitrary value is ordinarily one. For instance, set c_2 = 1 and solve the system of equations for the remaining coefficients: c_1 = 2 c_2 = 1 c_3 = 1 Substitute the coefficients into the chemical reaction to obtain the balanced equation: Answer: | | 2 + ⟶
Structures
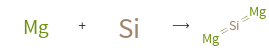
+ ⟶
Names
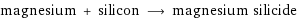
magnesium + silicon ⟶ magnesium silicide
Chemical names and formulas

| magnesium | silicon | magnesium silicide Hill formula | Mg | Si | Mg_2Si name | magnesium | silicon | magnesium silicide
Substance properties
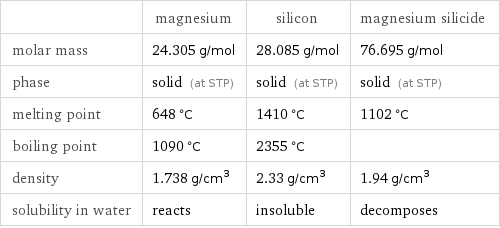
| magnesium | silicon | magnesium silicide molar mass | 24.305 g/mol | 28.085 g/mol | 76.695 g/mol phase | solid (at STP) | solid (at STP) | solid (at STP) melting point | 648 °C | 1410 °C | 1102 °C boiling point | 1090 °C | 2355 °C | density | 1.738 g/cm^3 | 2.33 g/cm^3 | 1.94 g/cm^3 solubility in water | reacts | insoluble | decomposes
Units
