Input interpretation
barium carbonate
Chemical names and formulas
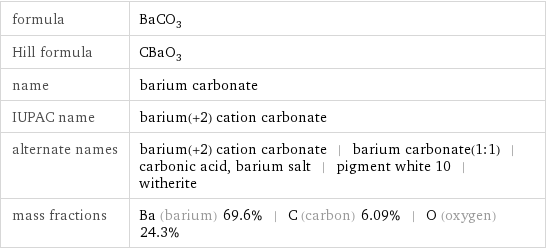
formula | BaCO_3 Hill formula | CBaO_3 name | barium carbonate IUPAC name | barium(+2) cation carbonate alternate names | barium(+2) cation carbonate | barium carbonate(1:1) | carbonic acid, barium salt | pigment white 10 | witherite mass fractions | Ba (barium) 69.6% | C (carbon) 6.09% | O (oxygen) 24.3%
Structure diagram
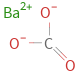
Structure diagram
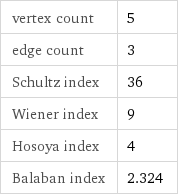
vertex count | 5 edge count | 3 Schultz index | 36 Wiener index | 9 Hosoya index | 4 Balaban index | 2.324
Basic properties
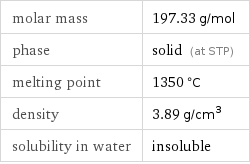
molar mass | 197.33 g/mol phase | solid (at STP) melting point | 1350 °C density | 3.89 g/cm^3 solubility in water | insoluble
Units

Solid properties (at STP)

density | 3.89 g/cm^3
Units

Thermodynamic properties
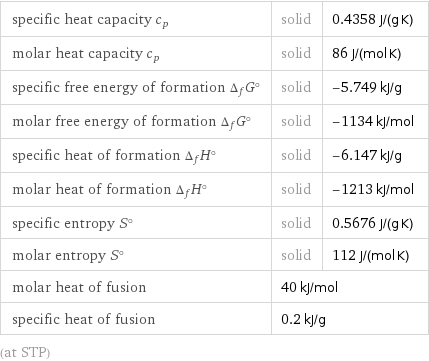
specific heat capacity c_p | solid | 0.4358 J/(g K) molar heat capacity c_p | solid | 86 J/(mol K) specific free energy of formation Δ_fG° | solid | -5.749 kJ/g molar free energy of formation Δ_fG° | solid | -1134 kJ/mol specific heat of formation Δ_fH° | solid | -6.147 kJ/g molar heat of formation Δ_fH° | solid | -1213 kJ/mol specific entropy S° | solid | 0.5676 J/(g K) molar entropy S° | solid | 112 J/(mol K) molar heat of fusion | 40 kJ/mol | specific heat of fusion | 0.2 kJ/g | (at STP)
Chemical identifiers
![CAS number | 513-77-9 Beilstein number | 7045119 PubChem CID number | 10563 PubChem SID number | 24852112 SMILES identifier | C(=O)([O-])[O-].[Ba+2] InChI identifier | InChI=1/CH2O3.Ba/c2-1(3)4;/h(H2, 2, 3, 4);/q;+2/p-2/fCO3.Ba/q-2;m EU number | 246-600-8 Gmelin number | 13524 RTECS number | CQ8600000 NSC number | 83508 MDL number | MFCD00003448](../image_source/f19f970175bfab02be9a89e520344d75.png)
CAS number | 513-77-9 Beilstein number | 7045119 PubChem CID number | 10563 PubChem SID number | 24852112 SMILES identifier | C(=O)([O-])[O-].[Ba+2] InChI identifier | InChI=1/CH2O3.Ba/c2-1(3)4;/h(H2, 2, 3, 4);/q;+2/p-2/fCO3.Ba/q-2;m EU number | 246-600-8 Gmelin number | 13524 RTECS number | CQ8600000 NSC number | 83508 MDL number | MFCD00003448
Toxicity properties

odor | odorless lethal dosage | 418 mg/kg (oral dose for rats)

probable lethal dose for man | 30 mL (milliliters)
Ion equivalents

Ba^(2+) (barium cation) | 1 (CO_3)^(2-) (carbonate anion) | 1