Input interpretation

period 3 elements | molar heat of vaporization
Summary
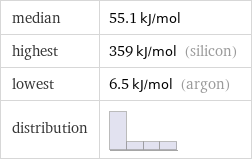
median | 55.1 kJ/mol highest | 359 kJ/mol (silicon) lowest | 6.5 kJ/mol (argon) distribution |
Units

Ranked values
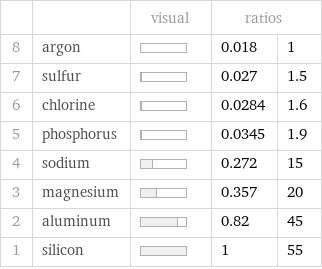
| | visual | ratios | 8 | argon | | 0.018 | 1 7 | sulfur | | 0.027 | 1.5 6 | chlorine | | 0.0284 | 1.6 5 | phosphorus | | 0.0345 | 1.9 4 | sodium | | 0.272 | 15 3 | magnesium | | 0.357 | 20 2 | aluminum | | 0.82 | 45 1 | silicon | | 1 | 55
Molar heat of vaporization rankings
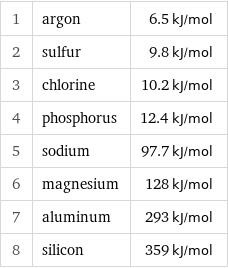
1 | argon | 6.5 kJ/mol 2 | sulfur | 9.8 kJ/mol 3 | chlorine | 10.2 kJ/mol 4 | phosphorus | 12.4 kJ/mol 5 | sodium | 97.7 kJ/mol 6 | magnesium | 128 kJ/mol 7 | aluminum | 293 kJ/mol 8 | silicon | 359 kJ/mol
Unit conversions for median molar heat of vaporization 55.1 kJ/mol
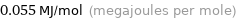
0.055 MJ/mol (megajoules per mole)
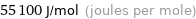
55100 J/mol (joules per mole)

13.2 kcal/mol (thermochemical kilocalories per mole)
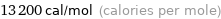
13200 cal/mol (calories per mole)

0.571 eV (molar electronvolts)