Input interpretation

cobalt(II) iodate | selenium tetrachloride
Chemical names and formulas
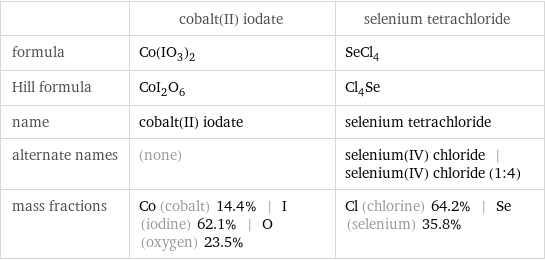
| cobalt(II) iodate | selenium tetrachloride formula | Co(IO_3)_2 | SeCl_4 Hill formula | CoI_2O_6 | Cl_4Se name | cobalt(II) iodate | selenium tetrachloride alternate names | (none) | selenium(IV) chloride | selenium(IV) chloride (1:4) mass fractions | Co (cobalt) 14.4% | I (iodine) 62.1% | O (oxygen) 23.5% | Cl (chlorine) 64.2% | Se (selenium) 35.8%
Structure diagrams
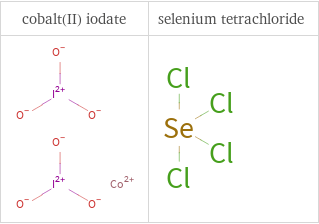
Structure diagrams
Basic properties
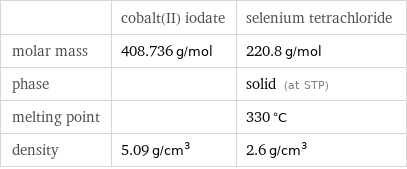
| cobalt(II) iodate | selenium tetrachloride molar mass | 408.736 g/mol | 220.8 g/mol phase | | solid (at STP) melting point | | 330 °C density | 5.09 g/cm^3 | 2.6 g/cm^3
Units

Solid properties

| selenium tetrachloride density | 2.6 g/cm^3 vapor pressure | 111.1 mmHg
Units
