Input interpretation
iodine trichloride
Chemical names and formulas
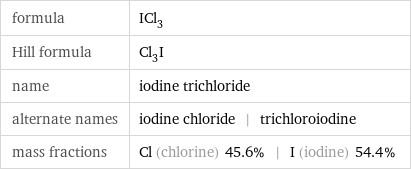
formula | ICl_3 Hill formula | Cl_3I name | iodine trichloride alternate names | iodine chloride | trichloroiodine mass fractions | Cl (chlorine) 45.6% | I (iodine) 54.4%
Lewis structure

Draw the Lewis structure of iodine trichloride. Start by drawing the overall structure of the molecule: Count the total valence electrons of the chlorine (n_Cl, val = 7) and iodine (n_I, val = 7) atoms: 3 n_Cl, val + n_I, val = 28 Calculate the number of electrons needed to completely fill the valence shells for chlorine (n_Cl, full = 8) and iodine (n_I, full = 8): 3 n_Cl, full + n_I, full = 32 Subtracting these two numbers shows that 32 - 28 = 4 bonding electrons are needed, which are already accounted for in the structure. Note that the valence shell of iodine has been expanded. After accounting for the expanded valence, there are 3 bonds and hence 6 bonding electrons in the diagram. Lastly, fill in the remaining unbonded electrons on each atom. In total, there remain 28 - 6 = 22 electrons left to draw: Answer: | |
Basic properties
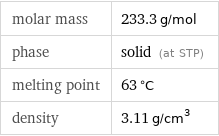
molar mass | 233.3 g/mol phase | solid (at STP) melting point | 63 °C density | 3.11 g/cm^3
Units

Solid properties (at STP)

density | 3.11 g/cm^3
Units

Chemical identifiers
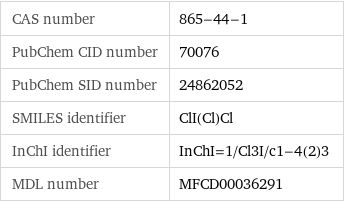
CAS number | 865-44-1 PubChem CID number | 70076 PubChem SID number | 24862052 SMILES identifier | ClI(Cl)Cl InChI identifier | InChI=1/Cl3I/c1-4(2)3 MDL number | MFCD00036291