Input interpretation
chloropentafluoroethane | hydrogen bromide
Chemical names and formulas
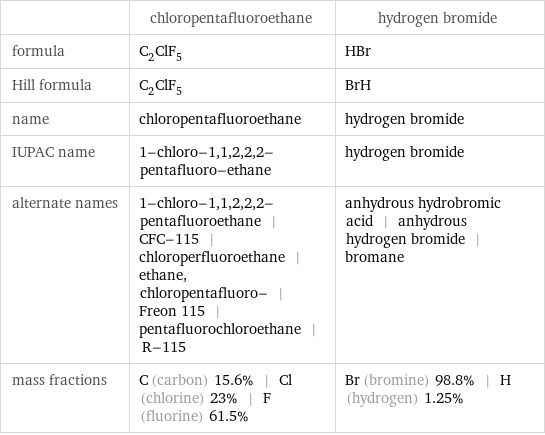
| chloropentafluoroethane | hydrogen bromide formula | C_2ClF_5 | HBr Hill formula | C_2ClF_5 | BrH name | chloropentafluoroethane | hydrogen bromide IUPAC name | 1-chloro-1, 1, 2, 2, 2-pentafluoro-ethane | hydrogen bromide alternate names | 1-chloro-1, 1, 2, 2, 2-pentafluoroethane | CFC-115 | chloroperfluoroethane | ethane, chloropentafluoro- | Freon 115 | pentafluorochloroethane | R-115 | anhydrous hydrobromic acid | anhydrous hydrogen bromide | bromane mass fractions | C (carbon) 15.6% | Cl (chlorine) 23% | F (fluorine) 61.5% | Br (bromine) 98.8% | H (hydrogen) 1.25%
Structure diagrams
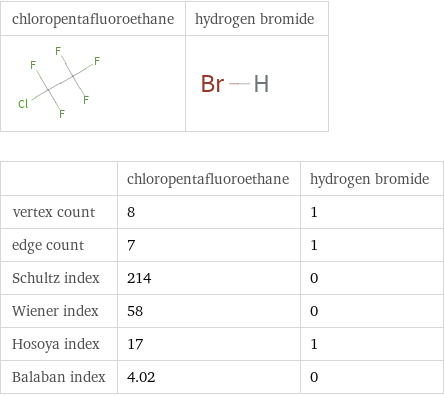
| chloropentafluoroethane | hydrogen bromide vertex count | 8 | 1 edge count | 7 | 1 Schultz index | 214 | 0 Wiener index | 58 | 0 Hosoya index | 17 | 1 Balaban index | 4.02 | 0
3D structure
3D structure
Basic properties
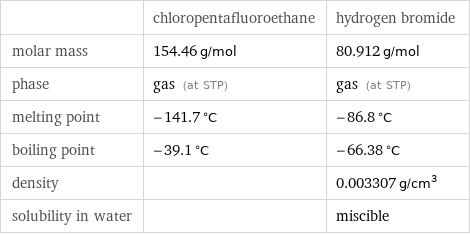
| chloropentafluoroethane | hydrogen bromide molar mass | 154.46 g/mol | 80.912 g/mol phase | gas (at STP) | gas (at STP) melting point | -141.7 °C | -86.8 °C boiling point | -39.1 °C | -66.38 °C density | | 0.003307 g/cm^3 solubility in water | | miscible
Units

Gas properties (at STP)
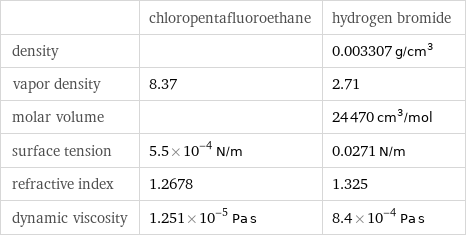
| chloropentafluoroethane | hydrogen bromide density | | 0.003307 g/cm^3 vapor density | 8.37 | 2.71 molar volume | | 24470 cm^3/mol surface tension | 5.5×10^-4 N/m | 0.0271 N/m refractive index | 1.2678 | 1.325 dynamic viscosity | 1.251×10^-5 Pa s | 8.4×10^-4 Pa s
Units

Liquid properties

| hydrogen bromide sound speed | 720 km/h
Units

Thermodynamic properties
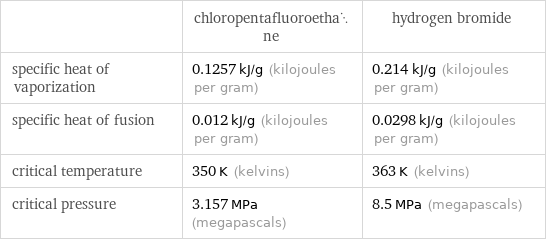
| chloropentafluoroethane | hydrogen bromide specific heat of vaporization | 0.1257 kJ/g (kilojoules per gram) | 0.214 kJ/g (kilojoules per gram) specific heat of fusion | 0.012 kJ/g (kilojoules per gram) | 0.0298 kJ/g (kilojoules per gram) critical temperature | 350 K (kelvins) | 363 K (kelvins) critical pressure | 3.157 MPa (megapascals) | 8.5 MPa (megapascals)