Input interpretation
manganese sulfide | calcium phosphonate monohydrate
Chemical names and formulas
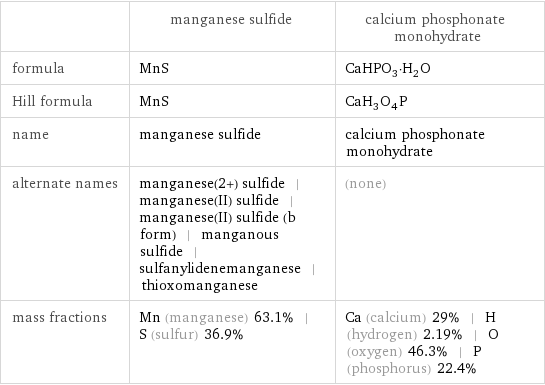
| manganese sulfide | calcium phosphonate monohydrate formula | MnS | CaHPO_3·H_2O Hill formula | MnS | CaH_3O_4P name | manganese sulfide | calcium phosphonate monohydrate alternate names | manganese(2+) sulfide | manganese(II) sulfide | manganese(II) sulfide (b form) | manganous sulfide | sulfanylidenemanganese | thioxomanganese | (none) mass fractions | Mn (manganese) 63.1% | S (sulfur) 36.9% | Ca (calcium) 29% | H (hydrogen) 2.19% | O (oxygen) 46.3% | P (phosphorus) 22.4%
Structure diagrams
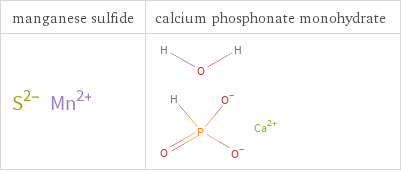
Structure diagrams
Basic properties
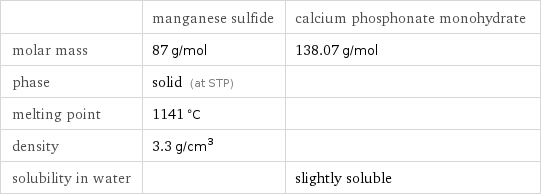
| manganese sulfide | calcium phosphonate monohydrate molar mass | 87 g/mol | 138.07 g/mol phase | solid (at STP) | melting point | 1141 °C | density | 3.3 g/cm^3 | solubility in water | | slightly soluble
Units
