Input interpretation
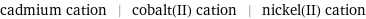
cadmium cation | cobalt(II) cation | nickel(II) cation
Structure diagrams
Structure diagrams
General properties
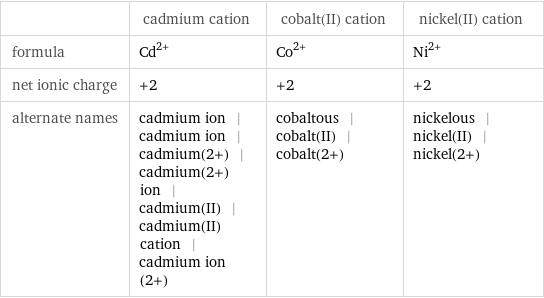
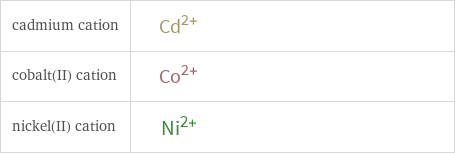
| cadmium cation | cobalt(II) cation | nickel(II) cation formula | Cd^(2+) | Co^(2+) | Ni^(2+) net ionic charge | +2 | +2 | +2 alternate names | cadmium ion | cadmium ion | cadmium(2+) | cadmium(2+) ion | cadmium(II) | cadmium(II) cation | cadmium ion (2+) | cobaltous | cobalt(II) | cobalt(2+) | nickelous | nickel(II) | nickel(2+)
Ionic radii
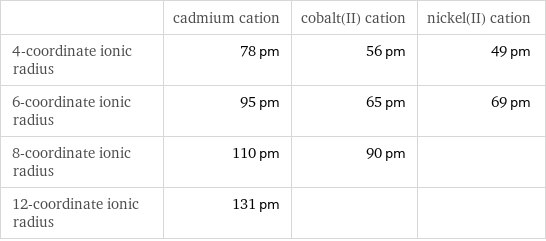
| cadmium cation | cobalt(II) cation | nickel(II) cation 4-coordinate ionic radius | 78 pm | 56 pm | 49 pm 6-coordinate ionic radius | 95 pm | 65 pm | 69 pm 8-coordinate ionic radius | 110 pm | 90 pm | 12-coordinate ionic radius | 131 pm | |
Units

Other properties
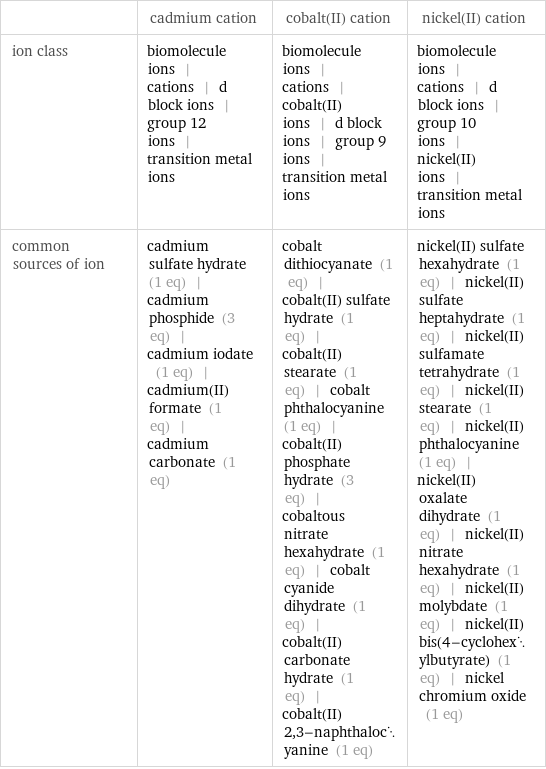
| cadmium cation | cobalt(II) cation | nickel(II) cation ion class | biomolecule ions | cations | d block ions | group 12 ions | transition metal ions | biomolecule ions | cations | cobalt(II) ions | d block ions | group 9 ions | transition metal ions | biomolecule ions | cations | d block ions | group 10 ions | nickel(II) ions | transition metal ions common sources of ion | cadmium sulfate hydrate (1 eq) | cadmium phosphide (3 eq) | cadmium iodate (1 eq) | cadmium(II) formate (1 eq) | cadmium carbonate (1 eq) | cobalt dithiocyanate (1 eq) | cobalt(II) sulfate hydrate (1 eq) | cobalt(II) stearate (1 eq) | cobalt phthalocyanine (1 eq) | cobalt(II) phosphate hydrate (3 eq) | cobaltous nitrate hexahydrate (1 eq) | cobalt cyanide dihydrate (1 eq) | cobalt(II) carbonate hydrate (1 eq) | cobalt(II) 2, 3-naphthalocyanine (1 eq) | nickel(II) sulfate hexahydrate (1 eq) | nickel(II) sulfate heptahydrate (1 eq) | nickel(II) sulfamate tetrahydrate (1 eq) | nickel(II) stearate (1 eq) | nickel(II) phthalocyanine (1 eq) | nickel(II) oxalate dihydrate (1 eq) | nickel(II) nitrate hexahydrate (1 eq) | nickel(II) molybdate (1 eq) | nickel(II) bis(4-cyclohexylbutyrate) (1 eq) | nickel chromium oxide (1 eq)