Input interpretation
helium (chemical element)
Periodic table location
Periodic table location
Image
Image
Basic elemental properties
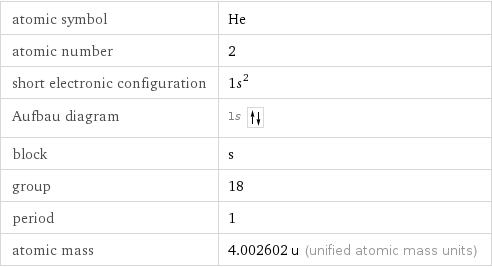
atomic symbol | He atomic number | 2 short electronic configuration | 1s^2 Aufbau diagram | 1s block | s group | 18 period | 1 atomic mass | 4.002602 u (unified atomic mass units)
Thermodynamic properties
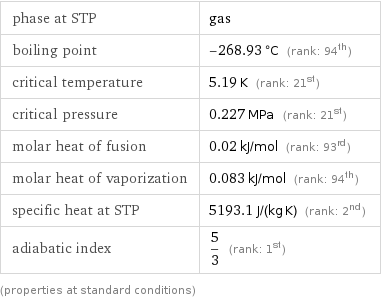
phase at STP | gas boiling point | -268.93 °C (rank: 94th) critical temperature | 5.19 K (rank: 21st) critical pressure | 0.227 MPa (rank: 21st) molar heat of fusion | 0.02 kJ/mol (rank: 93rd) molar heat of vaporization | 0.083 kJ/mol (rank: 94th) specific heat at STP | 5193.1 J/(kg K) (rank: 2nd) adiabatic index | 5/3 (rank: 1st) (properties at standard conditions)
Material properties
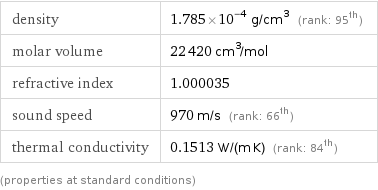
density | 1.785×10^-4 g/cm^3 (rank: 95th) molar volume | 22420 cm^3/mol refractive index | 1.000035 sound speed | 970 m/s (rank: 66th) thermal conductivity | 0.1513 W/(m K) (rank: 84th) (properties at standard conditions)
Electromagnetic properties
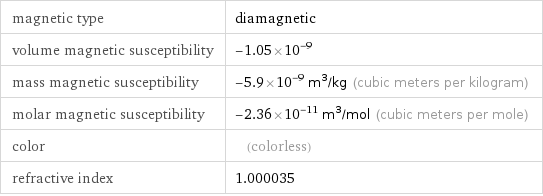
magnetic type | diamagnetic volume magnetic susceptibility | -1.05×10^-9 mass magnetic susceptibility | -5.9×10^-9 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | -2.36×10^-11 m^3/mol (cubic meters per mole) color | (colorless) refractive index | 1.000035
Reactivity
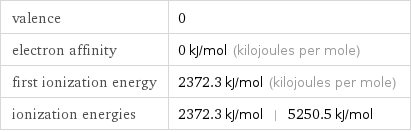
valence | 0 electron affinity | 0 kJ/mol (kilojoules per mole) first ionization energy | 2372.3 kJ/mol (kilojoules per mole) ionization energies | 2372.3 kJ/mol | 5250.5 kJ/mol
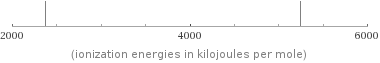
Reactivity
Atomic properties
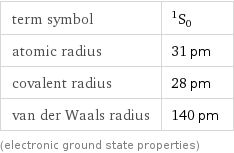
term symbol | ^1S_0 atomic radius | 31 pm covalent radius | 28 pm van der Waals radius | 140 pm (electronic ground state properties)
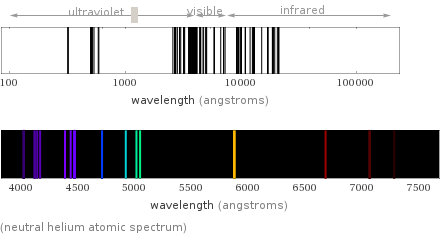
(neutral helium atomic spectrum)
Abundances
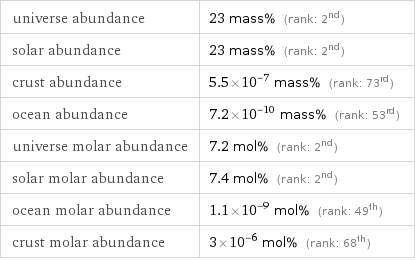
universe abundance | 23 mass% (rank: 2nd) solar abundance | 23 mass% (rank: 2nd) crust abundance | 5.5×10^-7 mass% (rank: 73rd) ocean abundance | 7.2×10^-10 mass% (rank: 53rd) universe molar abundance | 7.2 mol% (rank: 2nd) solar molar abundance | 7.4 mol% (rank: 2nd) ocean molar abundance | 1.1×10^-9 mol% (rank: 49th) crust molar abundance | 3×10^-6 mol% (rank: 68th)
Nuclear properties
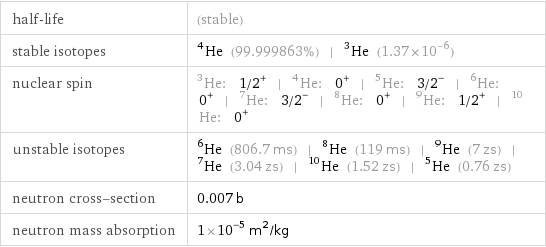
half-life | (stable) stable isotopes | He-4 (99.999863%) | He-3 (1.37×10^-6) nuclear spin | He-3: 1/2^+ | He-4: 0^+ | He-5: 3/2^- | He-6: 0^+ | He-7: 3/2^- | He-8: 0^+ | He-9: 1/2^+ | He-10: 0^+ unstable isotopes | He-6 (806.7 ms) | He-8 (119 ms) | He-9 (7 zs) | He-7 (3.04 zs) | He-10 (1.52 zs) | He-5 (0.76 zs) neutron cross-section | 0.007 b neutron mass absorption | 1×10^-5 m^2/kg
Identifiers

CAS number | 7440-59-7 PubChem CID number | 23987