Input interpretation
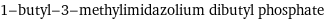
1-butyl-3-methylimidazolium dibutyl phosphate
Basic properties
![molar mass | 348.4 g/mol formula | C_16H_33N_2O_4P empirical formula | P_O_4C_16N_2H_33 SMILES identifier | CCCC[N+]1=CN(C)C=C1.CCCCOP(=O)([O-])OCCCC InChI identifier | InChI=1/C8H15N2.C8H19O4P/c1-3-4-5-10-7-6-9(2)8-10;1-3-5-7-11-13(9, 10)12-8-6-4-2/h6-8H, 3-5H2, 1-2H3;3-8H2, 1-2H3, (H, 9, 10)/q+1;/p-1/fC8H15N2.C8H18O4P/qm;-1 InChI key | NTXQJRGQUZXUMU-UHFFFAOYSA-M](../image_source/ee25ed92fe9e3a75e2eb19c033c63680.png)
molar mass | 348.4 g/mol formula | C_16H_33N_2O_4P empirical formula | P_O_4C_16N_2H_33 SMILES identifier | CCCC[N+]1=CN(C)C=C1.CCCCOP(=O)([O-])OCCCC InChI identifier | InChI=1/C8H15N2.C8H19O4P/c1-3-4-5-10-7-6-9(2)8-10;1-3-5-7-11-13(9, 10)12-8-6-4-2/h6-8H, 3-5H2, 1-2H3;3-8H2, 1-2H3, (H, 9, 10)/q+1;/p-1/fC8H15N2.C8H18O4P/qm;-1 InChI key | NTXQJRGQUZXUMU-UHFFFAOYSA-M
Structure diagram
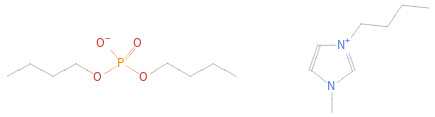
Structure diagram
Quantitative molecular descriptors
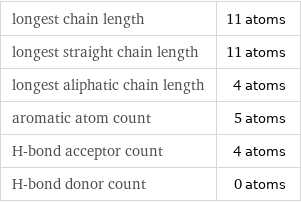
longest chain length | 11 atoms longest straight chain length | 11 atoms longest aliphatic chain length | 4 atoms aromatic atom count | 5 atoms H-bond acceptor count | 4 atoms H-bond donor count | 0 atoms
Elemental composition
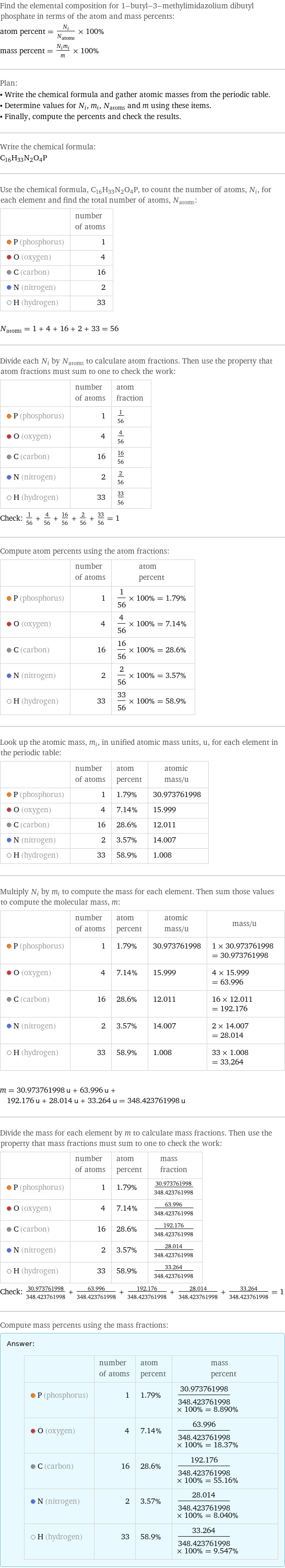
Find the elemental composition for 1-butyl-3-methylimidazolium dibutyl phosphate in terms of the atom and mass percents: atom percent = N_i/N_atoms × 100% mass percent = (N_im_i)/m × 100% Plan: • Write the chemical formula and gather atomic masses from the periodic table. • Determine values for N_i, m_i, N_atoms and m using these items. • Finally, compute the percents and check the results. Write the chemical formula: C_16H_33N_2O_4P Use the chemical formula, C_16H_33N_2O_4P, to count the number of atoms, N_i, for each element and find the total number of atoms, N_atoms: | number of atoms P (phosphorus) | 1 O (oxygen) | 4 C (carbon) | 16 N (nitrogen) | 2 H (hydrogen) | 33 N_atoms = 1 + 4 + 16 + 2 + 33 = 56 Divide each N_i by N_atoms to calculate atom fractions. Then use the property that atom fractions must sum to one to check the work: | number of atoms | atom fraction P (phosphorus) | 1 | 1/56 O (oxygen) | 4 | 4/56 C (carbon) | 16 | 16/56 N (nitrogen) | 2 | 2/56 H (hydrogen) | 33 | 33/56 Check: 1/56 + 4/56 + 16/56 + 2/56 + 33/56 = 1 Compute atom percents using the atom fractions: | number of atoms | atom percent P (phosphorus) | 1 | 1/56 × 100% = 1.79% O (oxygen) | 4 | 4/56 × 100% = 7.14% C (carbon) | 16 | 16/56 × 100% = 28.6% N (nitrogen) | 2 | 2/56 × 100% = 3.57% H (hydrogen) | 33 | 33/56 × 100% = 58.9% Look up the atomic mass, m_i, in unified atomic mass units, u, for each element in the periodic table: | number of atoms | atom percent | atomic mass/u P (phosphorus) | 1 | 1.79% | 30.973761998 O (oxygen) | 4 | 7.14% | 15.999 C (carbon) | 16 | 28.6% | 12.011 N (nitrogen) | 2 | 3.57% | 14.007 H (hydrogen) | 33 | 58.9% | 1.008 Multiply N_i by m_i to compute the mass for each element. Then sum those values to compute the molecular mass, m: | number of atoms | atom percent | atomic mass/u | mass/u P (phosphorus) | 1 | 1.79% | 30.973761998 | 1 × 30.973761998 = 30.973761998 O (oxygen) | 4 | 7.14% | 15.999 | 4 × 15.999 = 63.996 C (carbon) | 16 | 28.6% | 12.011 | 16 × 12.011 = 192.176 N (nitrogen) | 2 | 3.57% | 14.007 | 2 × 14.007 = 28.014 H (hydrogen) | 33 | 58.9% | 1.008 | 33 × 1.008 = 33.264 m = 30.973761998 u + 63.996 u + 192.176 u + 28.014 u + 33.264 u = 348.423761998 u Divide the mass for each element by m to calculate mass fractions. Then use the property that mass fractions must sum to one to check the work: | number of atoms | atom percent | mass fraction P (phosphorus) | 1 | 1.79% | 30.973761998/348.423761998 O (oxygen) | 4 | 7.14% | 63.996/348.423761998 C (carbon) | 16 | 28.6% | 192.176/348.423761998 N (nitrogen) | 2 | 3.57% | 28.014/348.423761998 H (hydrogen) | 33 | 58.9% | 33.264/348.423761998 Check: 30.973761998/348.423761998 + 63.996/348.423761998 + 192.176/348.423761998 + 28.014/348.423761998 + 33.264/348.423761998 = 1 Compute mass percents using the mass fractions: Answer: | | | number of atoms | atom percent | mass percent P (phosphorus) | 1 | 1.79% | 30.973761998/348.423761998 × 100% = 8.890% O (oxygen) | 4 | 7.14% | 63.996/348.423761998 × 100% = 18.37% C (carbon) | 16 | 28.6% | 192.176/348.423761998 × 100% = 55.16% N (nitrogen) | 2 | 3.57% | 28.014/348.423761998 × 100% = 8.040% H (hydrogen) | 33 | 58.9% | 33.264/348.423761998 × 100% = 9.547%
Elemental oxidation states
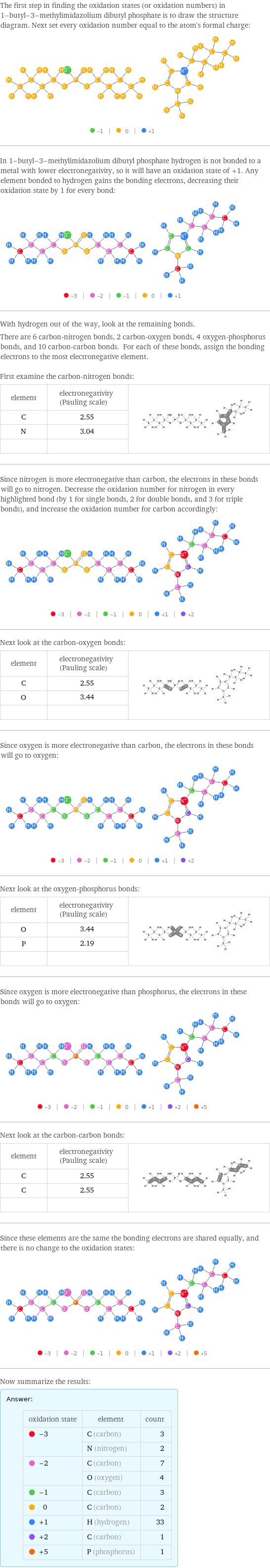
The first step in finding the oxidation states (or oxidation numbers) in 1-butyl-3-methylimidazolium dibutyl phosphate is to draw the structure diagram. Next set every oxidation number equal to the atom's formal charge: In 1-butyl-3-methylimidazolium dibutyl phosphate hydrogen is not bonded to a metal with lower electronegativity, so it will have an oxidation state of +1. Any element bonded to hydrogen gains the bonding electrons, decreasing their oxidation state by 1 for every bond: With hydrogen out of the way, look at the remaining bonds. There are 6 carbon-nitrogen bonds, 2 carbon-oxygen bonds, 4 oxygen-phosphorus bonds, and 10 carbon-carbon bonds. For each of these bonds, assign the bonding electrons to the most electronegative element. First examine the carbon-nitrogen bonds: element | electronegativity (Pauling scale) | C | 2.55 | N | 3.04 | | | Since nitrogen is more electronegative than carbon, the electrons in these bonds will go to nitrogen. Decrease the oxidation number for nitrogen in every highlighted bond (by 1 for single bonds, 2 for double bonds, and 3 for triple bonds), and increase the oxidation number for carbon accordingly: Next look at the carbon-oxygen bonds: element | electronegativity (Pauling scale) | C | 2.55 | O | 3.44 | | | Since oxygen is more electronegative than carbon, the electrons in these bonds will go to oxygen: Next look at the oxygen-phosphorus bonds: element | electronegativity (Pauling scale) | O | 3.44 | P | 2.19 | | | Since oxygen is more electronegative than phosphorus, the electrons in these bonds will go to oxygen: Next look at the carbon-carbon bonds: element | electronegativity (Pauling scale) | C | 2.55 | C | 2.55 | | | Since these elements are the same the bonding electrons are shared equally, and there is no change to the oxidation states: Now summarize the results: Answer: | | oxidation state | element | count -3 | C (carbon) | 3 | N (nitrogen) | 2 -2 | C (carbon) | 7 | O (oxygen) | 4 -1 | C (carbon) | 3 0 | C (carbon) | 2 +1 | H (hydrogen) | 33 +2 | C (carbon) | 1 +5 | P (phosphorus) | 1
Orbital hybridization
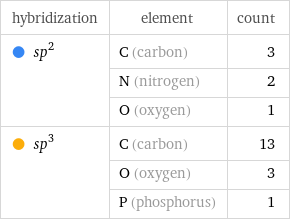
hybridization | element | count sp^2 | C (carbon) | 3 | N (nitrogen) | 2 | O (oxygen) | 1 sp^3 | C (carbon) | 13 | O (oxygen) | 3 | P (phosphorus) | 1
Structure diagram
Orbital hybridization Structure diagram
Topological indices
vertex count | 56 edge count | 55 Schultz index | Wiener index | Hosoya index | Balaban index |