Input interpretation
chlorine (chemical element) | gallium (chemical element) | bromine (chemical element)
Periodic table location
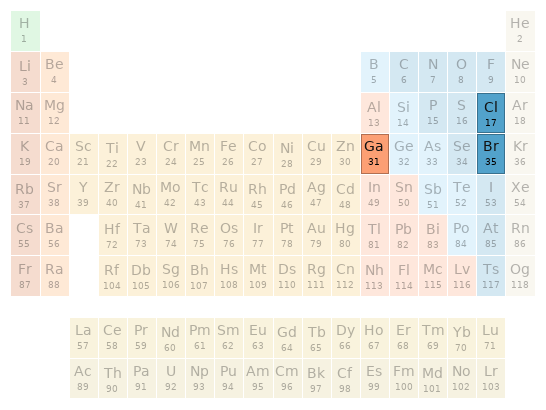
Periodic table location
Images
Images
Basic elemental properties
![| chlorine | gallium | bromine atomic symbol | Cl | Ga | Br atomic number | 17 | 31 | 35 short electronic configuration | [Ne]3s^23p^5 | [Ar]4s^23d^104p^1 | [Ar]4s^23d^104p^5 Aufbau diagram | 3p 3s | 4p 3d 4s | 4p 3d 4s block | p | p | p group | 17 | 13 | 17 period | 3 | 4 | 4 atomic mass | 35.45 u | 69.723 u | 79.904 u half-life | (stable) | (stable) | (stable)](../image_source/a57fa46681274dfcc0496d7957790ddb.png)
| chlorine | gallium | bromine atomic symbol | Cl | Ga | Br atomic number | 17 | 31 | 35 short electronic configuration | [Ne]3s^23p^5 | [Ar]4s^23d^104p^1 | [Ar]4s^23d^104p^5 Aufbau diagram | 3p 3s | 4p 3d 4s | 4p 3d 4s block | p | p | p group | 17 | 13 | 17 period | 3 | 4 | 4 atomic mass | 35.45 u | 69.723 u | 79.904 u half-life | (stable) | (stable) | (stable)
Thermodynamic properties
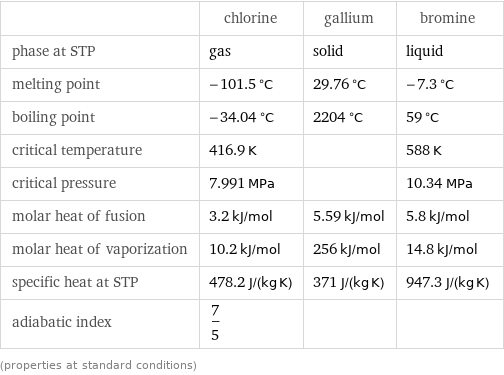
| chlorine | gallium | bromine phase at STP | gas | solid | liquid melting point | -101.5 °C | 29.76 °C | -7.3 °C boiling point | -34.04 °C | 2204 °C | 59 °C critical temperature | 416.9 K | | 588 K critical pressure | 7.991 MPa | | 10.34 MPa molar heat of fusion | 3.2 kJ/mol | 5.59 kJ/mol | 5.8 kJ/mol molar heat of vaporization | 10.2 kJ/mol | 256 kJ/mol | 14.8 kJ/mol specific heat at STP | 478.2 J/(kg K) | 371 J/(kg K) | 947.3 J/(kg K) adiabatic index | 7/5 | | (properties at standard conditions)
Material properties
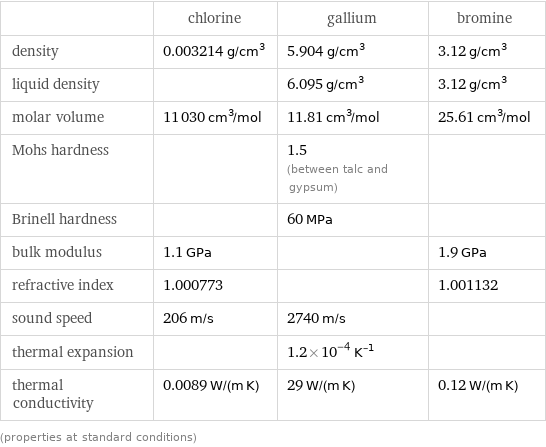
| chlorine | gallium | bromine density | 0.003214 g/cm^3 | 5.904 g/cm^3 | 3.12 g/cm^3 liquid density | | 6.095 g/cm^3 | 3.12 g/cm^3 molar volume | 11030 cm^3/mol | 11.81 cm^3/mol | 25.61 cm^3/mol Mohs hardness | | 1.5 (between talc and gypsum) | Brinell hardness | | 60 MPa | bulk modulus | 1.1 GPa | | 1.9 GPa refractive index | 1.000773 | | 1.001132 sound speed | 206 m/s | 2740 m/s | thermal expansion | | 1.2×10^-4 K^(-1) | thermal conductivity | 0.0089 W/(m K) | 29 W/(m K) | 0.12 W/(m K) (properties at standard conditions)
Electromagnetic properties

| chlorine | gallium | bromine electrical type | insulator | conductor | insulator resistivity | 100 Ω m (ohm meters) | 1.4×10^-7 Ω m (ohm meters) | 1×10^10 Ω m (ohm meters) electrical conductivity | 0.01 S/m (siemens per meter) | 7.1×10^6 S/m (siemens per meter) | 1×10^-10 S/m (siemens per meter) magnetic type | diamagnetic | diamagnetic | diamagnetic volume magnetic susceptibility | -2.31×10^-8 | -1.77×10^-5 | -1.53×10^-5 mass magnetic susceptibility | -7.2×10^-9 m^3/kg (cubic meters per kilogram) | -3×10^-9 m^3/kg (cubic meters per kilogram) | -4.9×10^-9 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | -5.11×10^-10 m^3/mol (cubic meters per mole) | -2.09×10^-10 m^3/mol (cubic meters per mole) | -7.83×10^-10 m^3/mol (cubic meters per mole) work function | | 4.32 eV (Polycrystalline) | threshold frequency | | 1.045×10^15 Hz (hertz) | superconducting point | | 1.083 K (kelvins) | color | (yellow) | (silver) | (red) refractive index | 1.000773 | | 1.001132
Reactivity
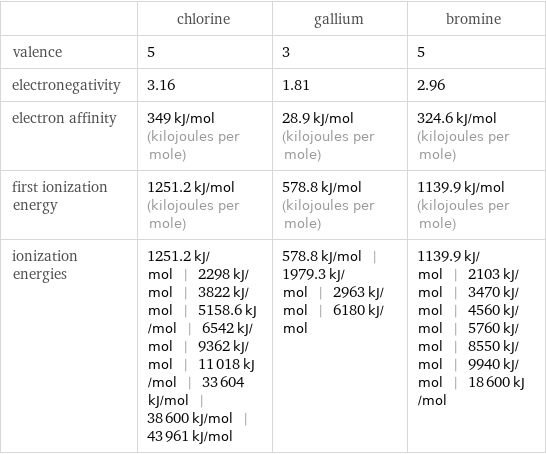
| chlorine | gallium | bromine valence | 5 | 3 | 5 electronegativity | 3.16 | 1.81 | 2.96 electron affinity | 349 kJ/mol (kilojoules per mole) | 28.9 kJ/mol (kilojoules per mole) | 324.6 kJ/mol (kilojoules per mole) first ionization energy | 1251.2 kJ/mol (kilojoules per mole) | 578.8 kJ/mol (kilojoules per mole) | 1139.9 kJ/mol (kilojoules per mole) ionization energies | 1251.2 kJ/mol | 2298 kJ/mol | 3822 kJ/mol | 5158.6 kJ/mol | 6542 kJ/mol | 9362 kJ/mol | 11018 kJ/mol | 33604 kJ/mol | 38600 kJ/mol | 43961 kJ/mol | 578.8 kJ/mol | 1979.3 kJ/mol | 2963 kJ/mol | 6180 kJ/mol | 1139.9 kJ/mol | 2103 kJ/mol | 3470 kJ/mol | 4560 kJ/mol | 5760 kJ/mol | 8550 kJ/mol | 9940 kJ/mol | 18600 kJ/mol
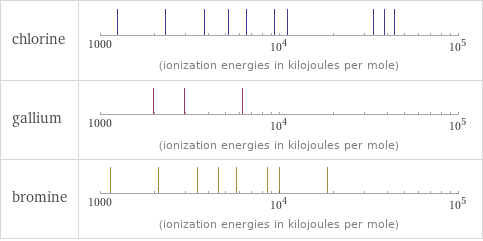
Reactivity
Atomic properties
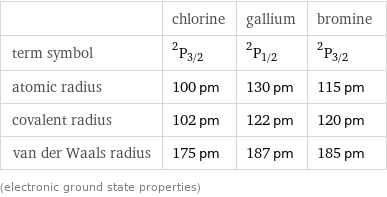
| chlorine | gallium | bromine term symbol | ^2P_(3/2) | ^2P_(1/2) | ^2P_(3/2) atomic radius | 100 pm | 130 pm | 115 pm covalent radius | 102 pm | 122 pm | 120 pm van der Waals radius | 175 pm | 187 pm | 185 pm (electronic ground state properties)
Abundances
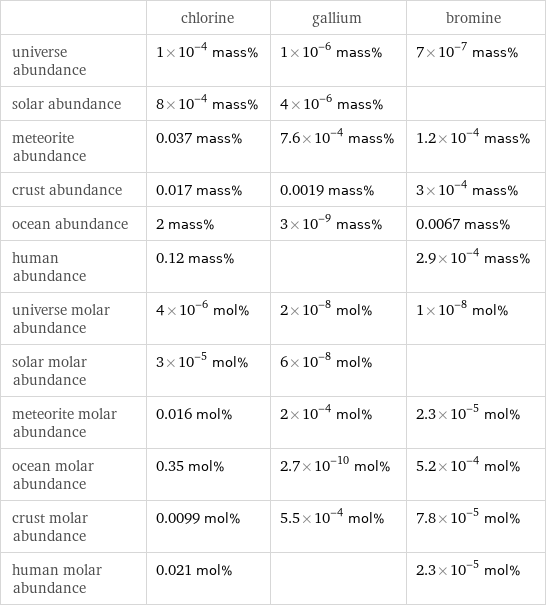
| chlorine | gallium | bromine universe abundance | 1×10^-4 mass% | 1×10^-6 mass% | 7×10^-7 mass% solar abundance | 8×10^-4 mass% | 4×10^-6 mass% | meteorite abundance | 0.037 mass% | 7.6×10^-4 mass% | 1.2×10^-4 mass% crust abundance | 0.017 mass% | 0.0019 mass% | 3×10^-4 mass% ocean abundance | 2 mass% | 3×10^-9 mass% | 0.0067 mass% human abundance | 0.12 mass% | | 2.9×10^-4 mass% universe molar abundance | 4×10^-6 mol% | 2×10^-8 mol% | 1×10^-8 mol% solar molar abundance | 3×10^-5 mol% | 6×10^-8 mol% | meteorite molar abundance | 0.016 mol% | 2×10^-4 mol% | 2.3×10^-5 mol% ocean molar abundance | 0.35 mol% | 2.7×10^-10 mol% | 5.2×10^-4 mol% crust molar abundance | 0.0099 mol% | 5.5×10^-4 mol% | 7.8×10^-5 mol% human molar abundance | 0.021 mol% | | 2.3×10^-5 mol%