Input interpretation

polar aprotic solvents | H-bond acceptor count
Summary
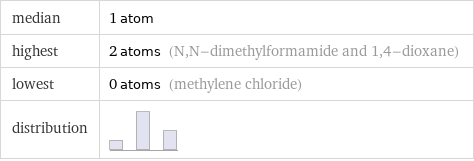
median | 1 atom highest | 2 atoms (N, N-dimethylformamide and 1, 4-dioxane) lowest | 0 atoms (methylene chloride) distribution |
H-bond acceptors in place
H-bond acceptors in place
H-bond acceptor count rankings
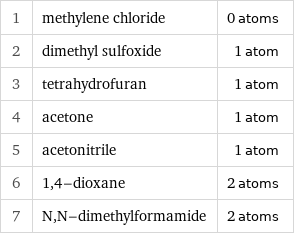
1 | methylene chloride | 0 atoms 2 | dimethyl sulfoxide | 1 atom 3 | tetrahydrofuran | 1 atom 4 | acetone | 1 atom 5 | acetonitrile | 1 atom 6 | 1, 4-dioxane | 2 atoms 7 | N, N-dimethylformamide | 2 atoms
H-bond acceptors elemental composition
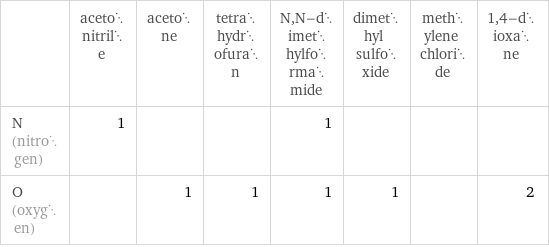
| acetonitrile | acetone | tetrahydrofuran | N, N-dimethylformamide | dimethyl sulfoxide | methylene chloride | 1, 4-dioxane N (nitrogen) | 1 | | | 1 | | | O (oxygen) | | 1 | 1 | 1 | 1 | | 2
Corresponding quantity
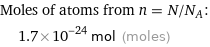
Moles of atoms from n = N/N_A: | 1.7×10^-24 mol (moles)