Input interpretation

6-ethoxy-2-naphthaleneboronic acid | molar mass
Result
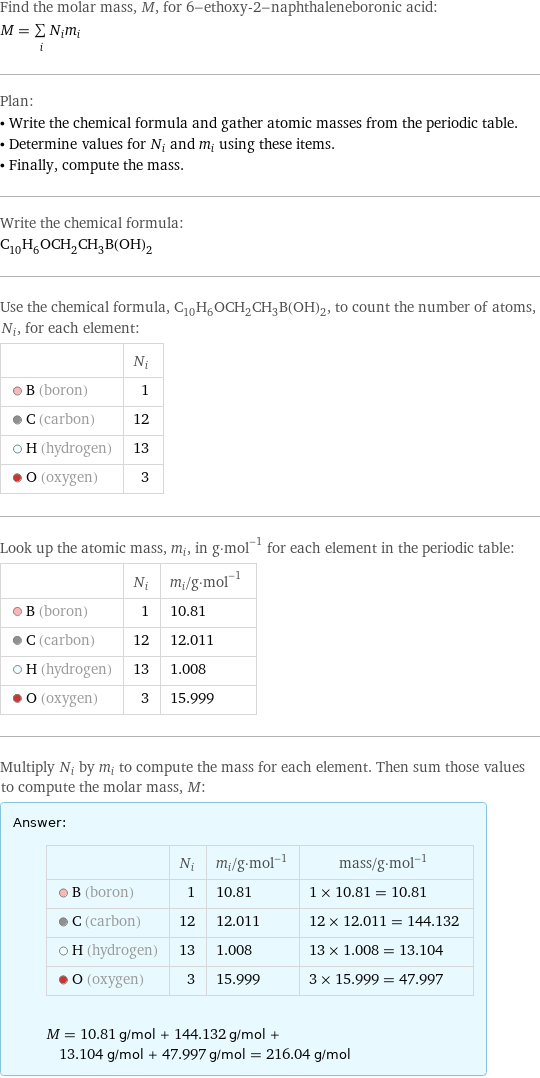
Find the molar mass, M, for 6-ethoxy-2-naphthaleneboronic acid: M = sum _iN_im_i Plan: • Write the chemical formula and gather atomic masses from the periodic table. • Determine values for N_i and m_i using these items. • Finally, compute the mass. Write the chemical formula: C_10H_6OCH_2CH_3B(OH)_2 Use the chemical formula, C_10H_6OCH_2CH_3B(OH)_2, to count the number of atoms, N_i, for each element: | N_i B (boron) | 1 C (carbon) | 12 H (hydrogen) | 13 O (oxygen) | 3 Look up the atomic mass, m_i, in g·mol^(-1) for each element in the periodic table: | N_i | m_i/g·mol^(-1) B (boron) | 1 | 10.81 C (carbon) | 12 | 12.011 H (hydrogen) | 13 | 1.008 O (oxygen) | 3 | 15.999 Multiply N_i by m_i to compute the mass for each element. Then sum those values to compute the molar mass, M: Answer: | | | N_i | m_i/g·mol^(-1) | mass/g·mol^(-1) B (boron) | 1 | 10.81 | 1 × 10.81 = 10.81 C (carbon) | 12 | 12.011 | 12 × 12.011 = 144.132 H (hydrogen) | 13 | 1.008 | 13 × 1.008 = 13.104 O (oxygen) | 3 | 15.999 | 3 × 15.999 = 47.997 M = 10.81 g/mol + 144.132 g/mol + 13.104 g/mol + 47.997 g/mol = 216.04 g/mol
Unit conversion
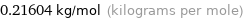
0.21604 kg/mol (kilograms per mole)
Comparisons
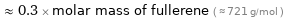
≈ 0.3 × molar mass of fullerene ( ≈ 721 g/mol )
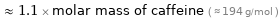
≈ 1.1 × molar mass of caffeine ( ≈ 194 g/mol )
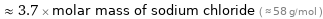
≈ 3.7 × molar mass of sodium chloride ( ≈ 58 g/mol )
Corresponding quantities
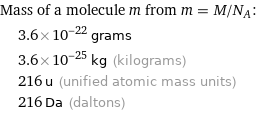
Mass of a molecule m from m = M/N_A: | 3.6×10^-22 grams | 3.6×10^-25 kg (kilograms) | 216 u (unified atomic mass units) | 216 Da (daltons)
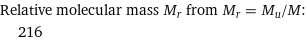
Relative molecular mass M_r from M_r = M_u/M: | 216