Input interpretation
carbon dioxide | hydrogen fluoride
Chemical names and formulas
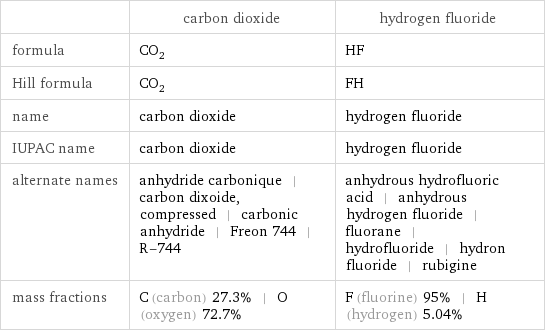
| carbon dioxide | hydrogen fluoride formula | CO_2 | HF Hill formula | CO_2 | FH name | carbon dioxide | hydrogen fluoride IUPAC name | carbon dioxide | hydrogen fluoride alternate names | anhydride carbonique | carbon dixoide, compressed | carbonic anhydride | Freon 744 | R-744 | anhydrous hydrofluoric acid | anhydrous hydrogen fluoride | fluorane | hydrofluoride | hydron fluoride | rubigine mass fractions | C (carbon) 27.3% | O (oxygen) 72.7% | F (fluorine) 95% | H (hydrogen) 5.04%
Structure diagrams

Structure diagrams
3D structure
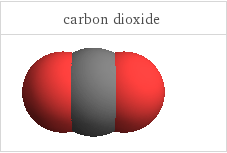
3D structure
Basic properties
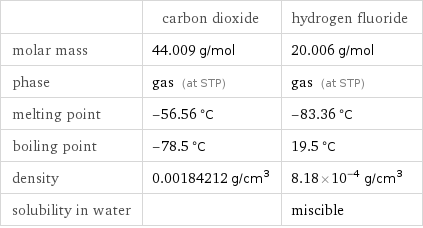
| carbon dioxide | hydrogen fluoride molar mass | 44.009 g/mol | 20.006 g/mol phase | gas (at STP) | gas (at STP) melting point | -56.56 °C | -83.36 °C boiling point | -78.5 °C | 19.5 °C density | 0.00184212 g/cm^3 | 8.18×10^-4 g/cm^3 solubility in water | | miscible
Units
