Input interpretation

liquid elements (at STP) | atomic mass
Results

bromine | 79.904 u (unified atomic mass units) mercury | 200.592 u (unified atomic mass units)
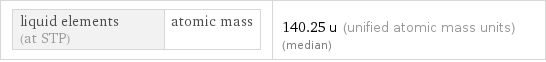
liquid elements (at STP) | atomic mass | 140.25 u (unified atomic mass units) (median)
Relative values

| visual | ratios | | comparisons bromine | | 0.39834 | 1 | 60.166% smaller mercury | | 1 | 2.5104 | 151.04% larger
Unit conversions for median atomic mass 140.25 u
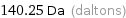
140.25 Da (daltons)
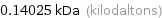
0.14025 kDa (kilodaltons)
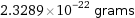
2.3289×10^-22 grams
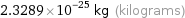
2.3289×10^-25 kg (kilograms)

140.25 chemical atomic mass units (unit officially deprecated)
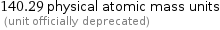
140.29 physical atomic mass units (unit officially deprecated)
Corresponding quantities
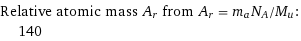
Relative atomic mass A_r from A_r = m_aN_A/M_u: | 140
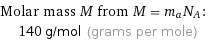
Molar mass M from M = m_aN_A: | 140 g/mol (grams per mole)