Input interpretation
sodium hydride
Chemical names and formulas
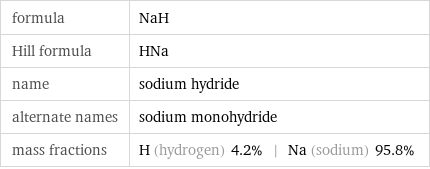
formula | NaH Hill formula | HNa name | sodium hydride alternate names | sodium monohydride mass fractions | H (hydrogen) 4.2% | Na (sodium) 95.8%
Structure diagram

Structure diagram
Basic properties
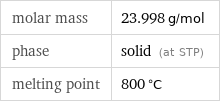
molar mass | 23.998 g/mol phase | solid (at STP) melting point | 800 °C
Units

Thermodynamic properties
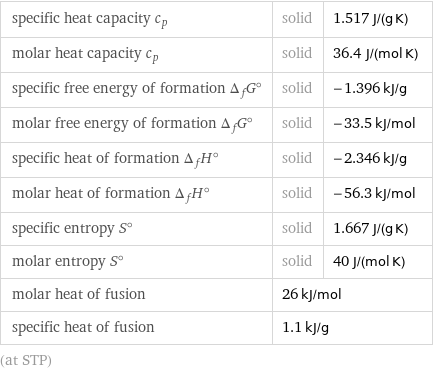
specific heat capacity c_p | solid | 1.517 J/(g K) molar heat capacity c_p | solid | 36.4 J/(mol K) specific free energy of formation Δ_fG° | solid | -1.396 kJ/g molar free energy of formation Δ_fG° | solid | -33.5 kJ/mol specific heat of formation Δ_fH° | solid | -2.346 kJ/g molar heat of formation Δ_fH° | solid | -56.3 kJ/mol specific entropy S° | solid | 1.667 J/(g K) molar entropy S° | solid | 40 J/(mol K) molar heat of fusion | 26 kJ/mol | specific heat of fusion | 1.1 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7646-69-7 PubChem CID number | 24758 PubChem SID number | 10535186 SMILES identifier | [H-].[Na+] InChI identifier | InChI=1/Na.H/q+1;-1 MDL number | MFCD00003471](../image_source/8e1f685b23178e282412f2de7cf71e59.png)
CAS number | 7646-69-7 PubChem CID number | 24758 PubChem SID number | 10535186 SMILES identifier | [H-].[Na+] InChI identifier | InChI=1/Na.H/q+1;-1 MDL number | MFCD00003471