Input interpretation
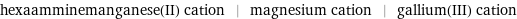
hexaamminemanganese(II) cation | magnesium cation | gallium(III) cation
Structure diagrams

Structure diagrams
General properties
![| hexaamminemanganese(II) cation | magnesium cation | gallium(III) cation formula | ([Mn(NH_3)_6])^(2+) | Mg^(2+) | Ga^(3+) net ionic charge | +2 | +2 | +3 alternate names | hexaamminemanganese(II) | hexaamminemanganese(2+) | magnesium ion | magnesium(2+) | gallium(III) | gallium(3+)](../image_source/12505d5787bbf47781be60c3042da4cd.png)
| hexaamminemanganese(II) cation | magnesium cation | gallium(III) cation formula | ([Mn(NH_3)_6])^(2+) | Mg^(2+) | Ga^(3+) net ionic charge | +2 | +2 | +3 alternate names | hexaamminemanganese(II) | hexaamminemanganese(2+) | magnesium ion | magnesium(2+) | gallium(III) | gallium(3+)
Ionic radii

| hexaamminemanganese(II) cation | magnesium cation | gallium(III) cation 4-coordinate ionic radius | | 57 pm | 47 pm 6-coordinate ionic radius | | 72 pm | 62 pm 8-coordinate ionic radius | | 89 pm | thermochemical radius | 265 pm | |
Units

Other properties
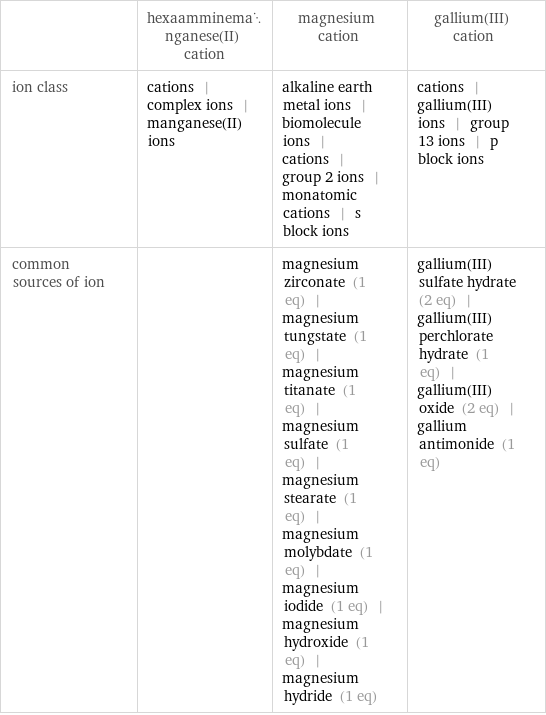
| hexaamminemanganese(II) cation | magnesium cation | gallium(III) cation ion class | cations | complex ions | manganese(II) ions | alkaline earth metal ions | biomolecule ions | cations | group 2 ions | monatomic cations | s block ions | cations | gallium(III) ions | group 13 ions | p block ions common sources of ion | | magnesium zirconate (1 eq) | magnesium tungstate (1 eq) | magnesium titanate (1 eq) | magnesium sulfate (1 eq) | magnesium stearate (1 eq) | magnesium molybdate (1 eq) | magnesium iodide (1 eq) | magnesium hydroxide (1 eq) | magnesium hydride (1 eq) | gallium(III) sulfate hydrate (2 eq) | gallium(III) perchlorate hydrate (1 eq) | gallium(III) oxide (2 eq) | gallium antimonide (1 eq)