Input interpretation
hydrogen iodide | chromic acid
Chemical names and formulas
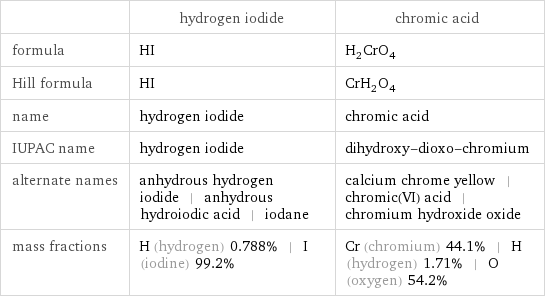
| hydrogen iodide | chromic acid formula | HI | H_2CrO_4 Hill formula | HI | CrH_2O_4 name | hydrogen iodide | chromic acid IUPAC name | hydrogen iodide | dihydroxy-dioxo-chromium alternate names | anhydrous hydrogen iodide | anhydrous hydroiodic acid | iodane | calcium chrome yellow | chromic(VI) acid | chromium hydroxide oxide mass fractions | H (hydrogen) 0.788% | I (iodine) 99.2% | Cr (chromium) 44.1% | H (hydrogen) 1.71% | O (oxygen) 54.2%
Structure diagrams
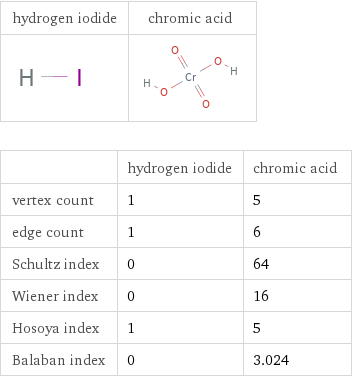
| hydrogen iodide | chromic acid vertex count | 1 | 5 edge count | 1 | 6 Schultz index | 0 | 64 Wiener index | 0 | 16 Hosoya index | 1 | 5 Balaban index | 0 | 3.024
3D structure
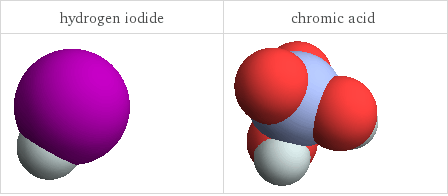
3D structure
Basic properties
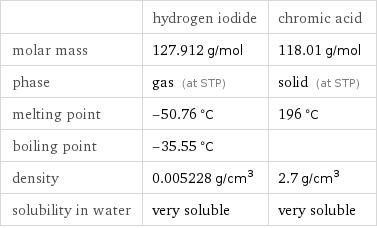
| hydrogen iodide | chromic acid molar mass | 127.912 g/mol | 118.01 g/mol phase | gas (at STP) | solid (at STP) melting point | -50.76 °C | 196 °C boiling point | -35.55 °C | density | 0.005228 g/cm^3 | 2.7 g/cm^3 solubility in water | very soluble | very soluble
Units

Gas properties
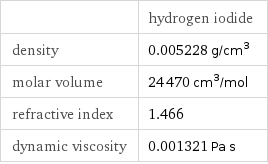
| hydrogen iodide density | 0.005228 g/cm^3 molar volume | 24470 cm^3/mol refractive index | 1.466 dynamic viscosity | 0.001321 Pa s
Units

Liquid properties

| hydrogen iodide sound speed | 565.2 km/h
Units

Thermodynamic properties
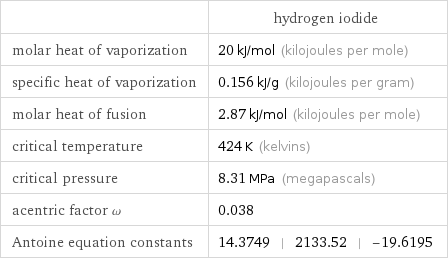
| hydrogen iodide molar heat of vaporization | 20 kJ/mol (kilojoules per mole) specific heat of vaporization | 0.156 kJ/g (kilojoules per gram) molar heat of fusion | 2.87 kJ/mol (kilojoules per mole) critical temperature | 424 K (kelvins) critical pressure | 8.31 MPa (megapascals) acentric factor ω | 0.038 Antoine equation constants | 14.3749 | 2133.52 | -19.6195