Input interpretation
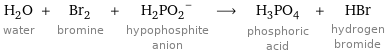
H_2O water + Br_2 bromine + (H_2PO_2)^- hypophosphite anion ⟶ H_3PO_4 phosphoric acid + HBr hydrogen bromide
Sample reactions
(data not available)
Chemical names and formulas
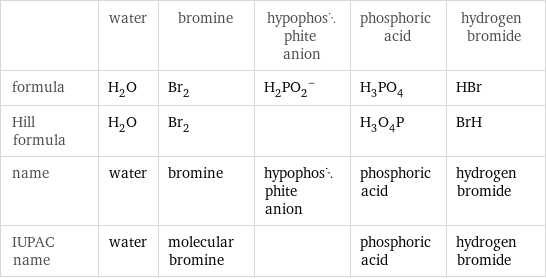
| water | bromine | hypophosphite anion | phosphoric acid | hydrogen bromide formula | H_2O | Br_2 | (H_2PO_2)^- | H_3PO_4 | HBr Hill formula | H_2O | Br_2 | | H_3O_4P | BrH name | water | bromine | hypophosphite anion | phosphoric acid | hydrogen bromide IUPAC name | water | molecular bromine | | phosphoric acid | hydrogen bromide