Input interpretation
lanthanum(III) cation
Structure diagram

Structure diagram
General properties

formula | La^(3+) net ionic charge | +3 alternate names | lanthanum(III) | lanthanum(3+)
Ionic radii
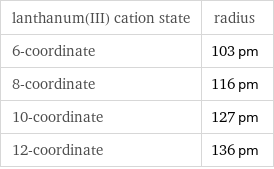
lanthanum(III) cation state | radius 6-coordinate | 103 pm 8-coordinate | 116 pm 10-coordinate | 127 pm 12-coordinate | 136 pm
Units

Other properties

ion class | cations | f block ions | lanthanide ions common sources of ion | lanthanum(III) sulfate nonahydrate (2 eq) | lanthanum(III) sulfate (2 eq) | lanthanum(III) phosphate hydrate (1 eq) | lanthanum(III) oxalate hydrate (2 eq) | lanthanum(III) nitrate hexahydrate (1 eq)
Thermodynamic properties
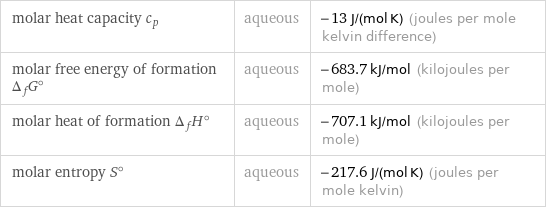
molar heat capacity c_p | aqueous | -13 J/(mol K) (joules per mole kelvin difference) molar free energy of formation Δ_fG° | aqueous | -683.7 kJ/mol (kilojoules per mole) molar heat of formation Δ_fH° | aqueous | -707.1 kJ/mol (kilojoules per mole) molar entropy S° | aqueous | -217.6 J/(mol K) (joules per mole kelvin)